Annals of Neurosciences, Volume 20, Issue 3 (July), 2013
The Substitute Brain and the Potential of the Gel Model
ABSTRACT |
This purpose of this paper is to review the recent history of the use of agarose gels. Although originally confined to electrophoresis work, agarose gels have proven themselves useful to a number of disciplines in the modern world, which includes brain infusion studies for research involving the treatment of various neurological conditions, such as Parkinson’s Disease. In reviewing the relevant research leading up to the modern day, this paper attempts to track agarose gels through their stages of accuracy verification, highlighting why they are useful to the neurosurgery discipline and characterizing the nature of their use. Agarose gels do have significant limitations, which are also discussed, but they have substantial potential as a modifiable medium or as a basis of comparison for even more accurate models in the future. KEYWORDS: Agarose gel, Brain infusion, In vitro model, Catheter, Protocol, History, Overview Corresponding Author: Roland Pomfret, PhD, Department of Neurological Surgery, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA Tel: (715)-410-6678, E-mail: pomfret@wisc.edu doi : 10.5214/ans.0972.7531.200309 |
Introduction
Gels are used as in vitro models in studies across numerous disciplines, including imaging, radiotherapy, and infusion studies for the treatment of debilitating neurological diseases, such as Parkinson’s Disease or brain cancer. Agarose gels have proven useful in these and many other neurosurgical applications.1 Low concentration agarose gel models are widely considered to be a viable in vitro model of the physical characteristics of the human brain. Low concentration agarose gels have been shown to accurately emulate the poroelasticity of the brain, a primary factor in its usefulness as a model for infusion studies.2 Poroelasticity has an effect on nearly every measured aspect of infusion, including pressure, backflow, and volume of distribution to volume of infusate ratios. In addition, their translucent nature allows for easy monitoring and the use of video recording as a form of data acquisition.3 The advantages of agarose gels are clear, but little work has been done to characterize its use. The purpose of this paper is to briefly explore the history of agarose gels in infusion studies in order to provide understanding for why they are so commonly used, to highlight the properties and characteristics that make them useful, and to describe the goals and concerns involved with agarose infusion models in the modern day.
History before utilization in infusion
A PubMed search of “agarose gel” indicates that it was first presented to the research world in the early 1960s as a medium for electrophoresis and/or chromatography. One of the earliest papers for which an abstract was available was Margolis’, use of 4% and 6% agarose gel in the size determination of lipoproteins, for which “agarose gel filtration shows promise as a useful method for the isolation, purification, and characterization of lipoproteins.”4 Agarose gel was not, however, only limited to lipoproteins. Other articles surfaced utilizing agarose gel for the well-known purpose of RNA and DNA electrophoresis, one of the earliest for which an abstract was being available Arlinghaus et al.5 The use of agarose gel as a diffusive medium greatly expanded beginning in the mid-1970s. For a medium capable of supporting the diffusion of nucleic acids or proteins, it is attractive to reason its to see the applicability in the diffusion and distribution of an infusate.
Characterizing elasticity and use as a model
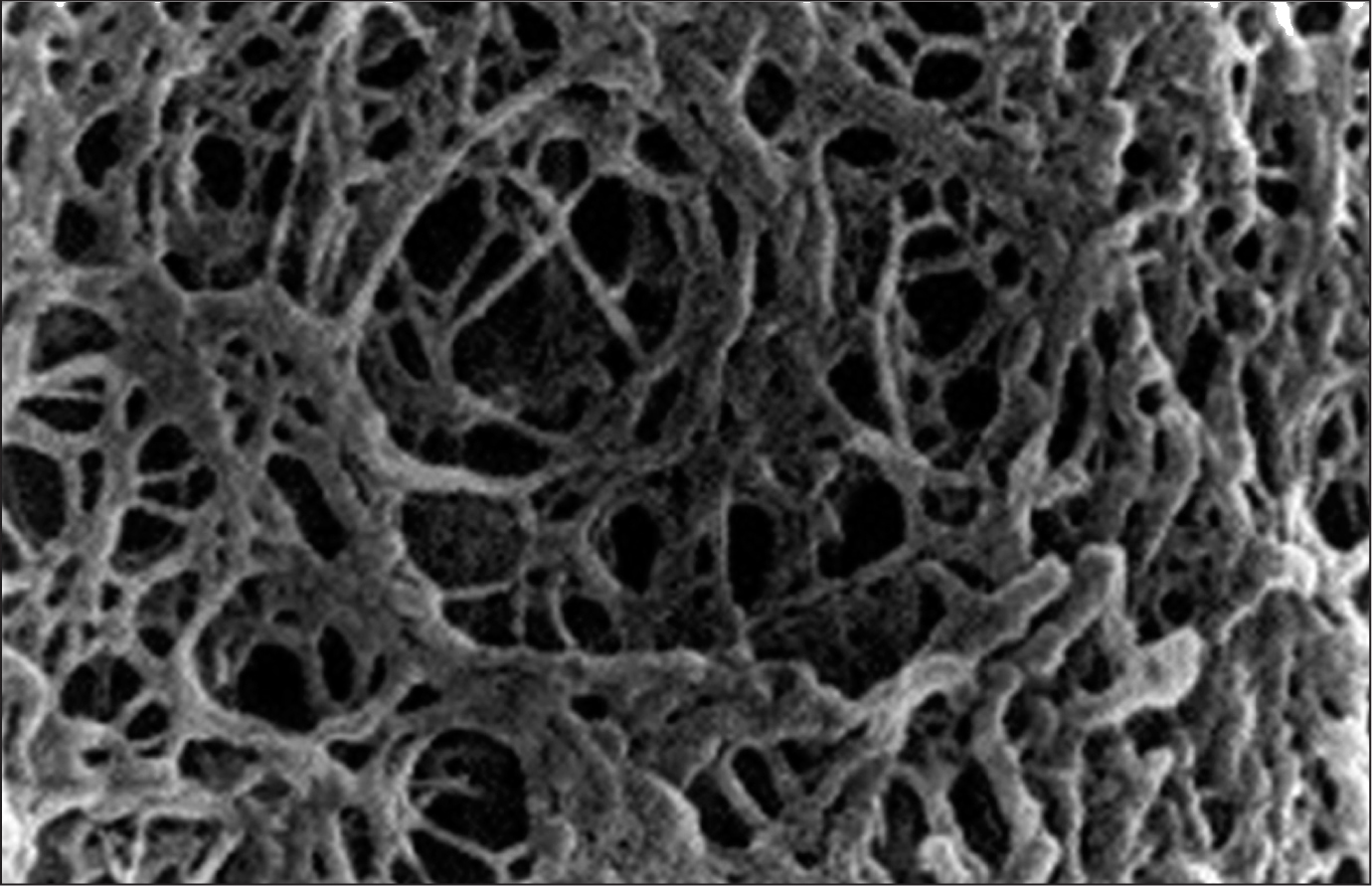
Before agarose gels could be utilized in modern infusion studies, the properties underlying its accuracy had to be verified. Although Fujii et al. did not relate their study on elasticity to infusion, this work, and others like it, are pivotal to the versatile application of agarose gel.6 Agarose gel is best envisioned as a web of fibersthat form cross-links as fibers overlap (Figure 1). Fujii et al. found that the average distance between cross-links decreased and the maximum shear stress supported by the gel increased with corresponding increase agarose gel concentration.6 This shows that with the increase in concentration of agarose its fiber density also increases, which has a large effect on elasticity.

Fig. 1: Microscopic Image of Agarose Gel Structure. Notice the web-like nature formed by cross-links as the gel ages and cools. Source: http://genetics.thetech.org/ask-a-geneticist/telling-2-dna-samples-apart. Accessed January 15, 2013.
The importance of this lies in the scope of an in vitro model of the brain which needs to be adjustable. At this time, little work had been performed to determine the parameters that an in vitro model of the brain must have in order to serve as an accurate surrogate. In order to find these parameters, future researchers interested in in vitro models of the brain needed a tool that they could adjust and then compare to an in vivo study in order to further hone the model’s accuracy. Due to the effects witnessed by a simple change in concentration, an adjustable agarose gel model proved to be one such tool.
Around this time, the use of gels for infusion studies was beginning to gather interest. One of the first infusion studies to use the gel model involved the infusion of the lambda phage virus into a 0.2% agarose gel, performed by Jilla et al. in 1999.7 0.2% was defended as an appropriate model out of numerous other gel concentrations due to its “transparency and structural characteristics.” In addition, Jilla et al. reported that 0.2% agarose gel had an infusion pressure profile similar to previous in vivo work.7 Later, Chen et al. used a similar agar model to investigate the infusion of tumor cells in order to test catheter designs and optimize them for CNS cell delivery.8 Agar gel was defended as an accurate model due to its portrayal of the brain’s “microscale characteristics of parenchymal tissues.”Chen et al. performed another important comparison of the in vitro model against a separate in vivo study concerning catheter drag force during insertion into the brain.2 Ahmed et al. proposed in vitro drag force gel experiment which was compared against proposal of Howard et al.’s in vivo drag force experiment.9,10 Ahmed et al used a 0.2% agarose gel, while Howard et al used brain tissue removed from epileptic patients. Both studies inserted a 3 mm ventricular catheter into their respective tissue at 0.33 mm/sec.9,10 The measured drag for the in vitro model was much lower, but within a factor of seven of that for the in vivo model. This information served to validate the model’s clinical validity.2
This verification demonstrates that while 0.2% gel may have been far from an ideal model, agarose gels which were capable of garnering relevant measurements of drag forces encountered by catheters upon insertion. This comparison would seem to support that a higher concentration, such as 0.6%, maybe more appropriate as an accurate model for the brain. A thicker, more viscous gel could probably exert the greater physical resistance required to produce a higher drag force. Chen et al. confirmed this notion by demonstrating that the force profile during insertion for 0.6% agarose gel and the porcine brain are remarkably similar.1 It should be noted that insertion force in vivo varies dramatically due to the non-homogeneity of the brain (Figure 4). Agarose at a specific concentration is homogenous, so perhaps multiple concentrations in layers would be required to accurately model the brain.
Acceptance of low concentration gels as accurate models
By the time that Gillies et al. published his June 2002 research article on the infusion of nanoparticles into gels in order to characterize their pore structure, 0.6% was considered to be an agarose gel concentration that accurately modeled the mammalian brain.11 According to Gillies et al., at this concentration, the porosity of the gel created by its crosslink structure closely resembles the porosity of the brain’s extracellular fluid. After discussing that 0.6% agarose gels are suitable for convection-enhanced delivery infusions in addition to electrophoretic transport phenomenon, Gillies et al. went showed the same gel model could be designed to create a spinal cord surrogate.12 The surrogate used non-gelatinous fibers encased in 0.6% agarose gel to mimic the fibrous neural tissue observed in some areas of the CNS. These fibres created “superpores,” which caused infusate to spread longitudinally rather than of spherically as it typically does (Figure 2). This observation matched closely with what was observed in in vivo studies of fibrous tissue in the CNS, which reveals another useful quality of agarose gels: they are easily modified. Physical and even chemical additions can be made to agarose gel in order to enhance the operationalization of the in vitro model, depending on what is being studied. This greatly expands its versatility and is part of the reason agarose models are still considered viable today.
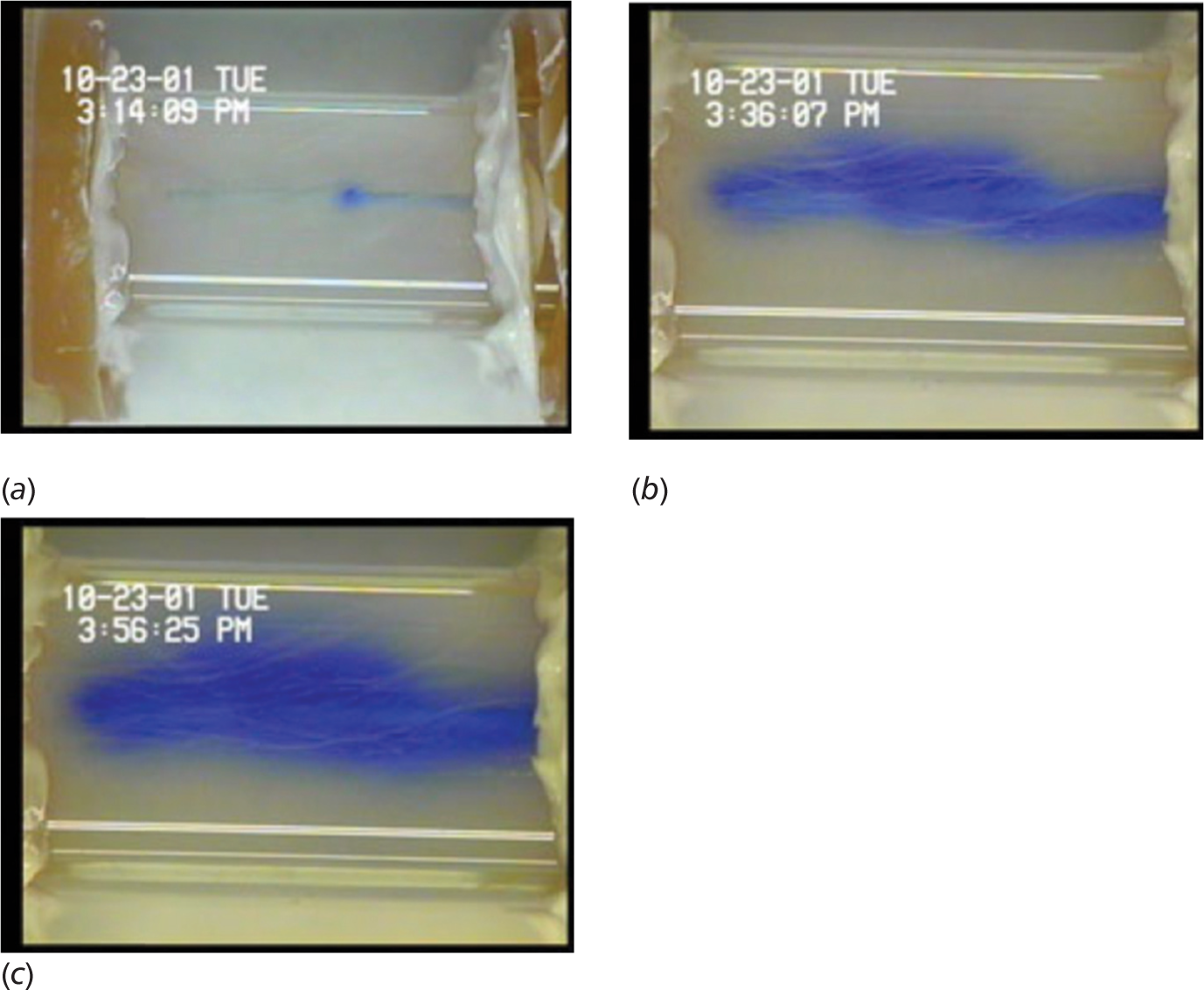
Fig. 2: Taken from “Gillies GT, Wilhelm TD, Humphrey JAC, Fillmore HL, Holloway KL, Broaddus WC. A spinal cord surrogate with nanoscale porosity for in vitro simulations of restorative neurosurgical techniques. Nanotechnology. 2002; 13(5): 587–91.” © IOP Publishing. Reproduced with permission. All rights reserved.
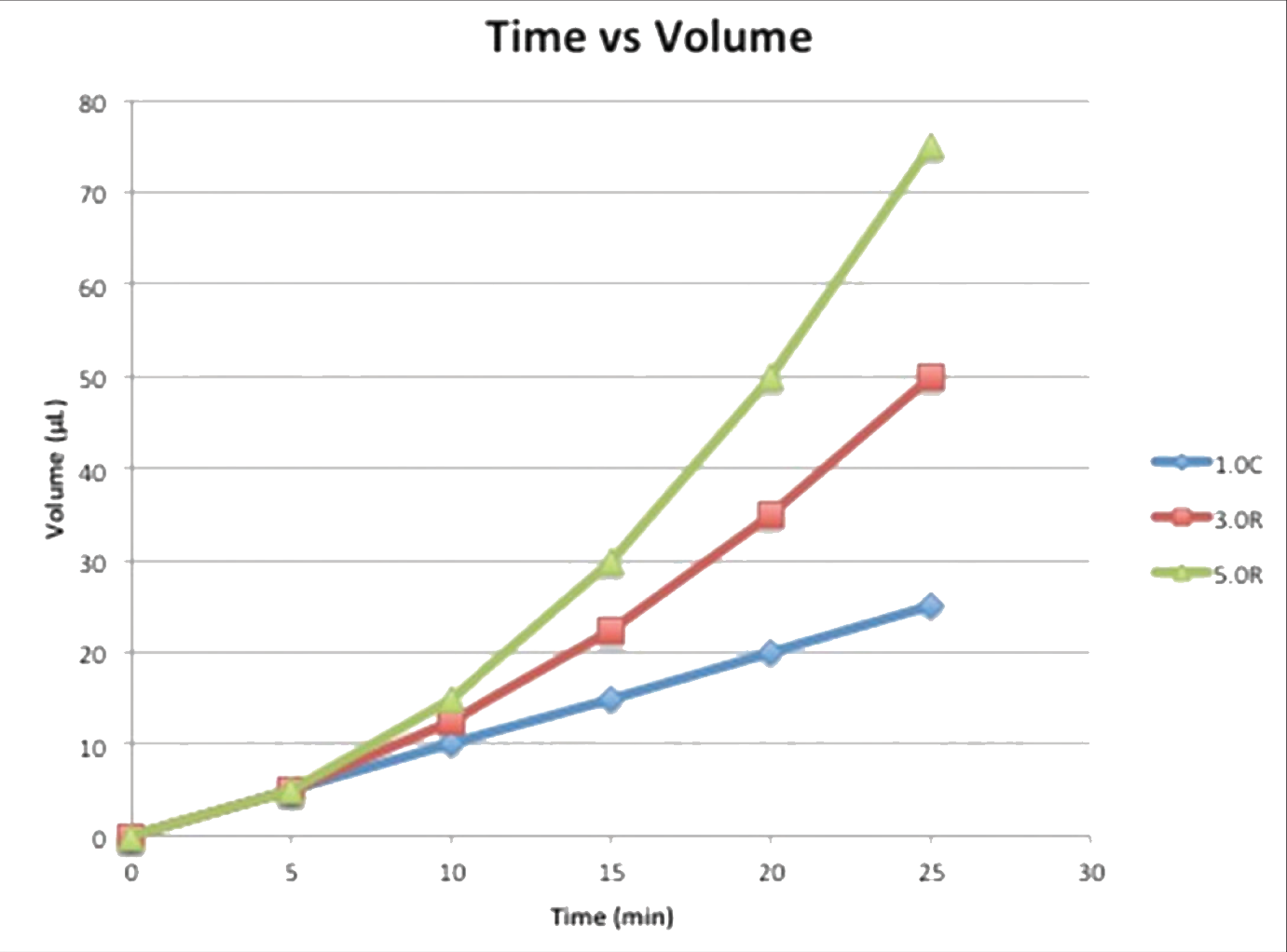
Fig. 3: In Sillay et al. (2012), bromophenol blue dye was injected in 0.2% agarose gel using the following protocols: 1.0C UW - Continuous infusion at 1.0 μL/min for 25 minutes; final volume of 25 μL. 3.0R UCSF - Ramped infusion at 1.0-3.0 μL/min at 0.5μL steps every 5 minutes for 25 minute final volume of 50 μL. 5.0R UCSF - Ramped infusion at 1.0–5.0 μL/min at 1.0μL steps every 5 minutes for 25 minutes; final volume of 75 μL. The depicted graph shows the volume infused over time for these three protocols. These protocols were tested using an ERG valve-tip catheter (VT) and an MRI Interventions Smart Flow (SF) catheter, shown in the picture.
Chen et al. showed a 0.2% agarose gel model could be explored as a viable option for modeling poroelasticity in the brain. One of the primary rationales for the use of 0.2% was that high molecular weight dyes, such as Blue Dextran, could actually be infused into the gel at this concentration. Aside from that, the only basis for the use of 0.2% over any other concentration was its use in previous work, specifically that of Jilla et al.7Gillies et al. 2002 reported quantitative evidence that 0.6% agarose gel was ideal for brain infusion studies, something that had not been done for 0.2% gel.11 In 2004, Chen et al. published a work comparing 0.6% gel, 0.8% gel, and the porcine brain across numerous different factors important to infusion, including infusion pressure, volume of distribution, and insertion pressure. Interestingly, 0.2% gel was not included in the study, but the collected data between the pig brain and that of 0.6% gel were strikingly similar, with profiles for 0.6% gel being only slightly higher than those of the porcine brain for all categories.1 The author was unable to locate any studies directly comparing 0.2% gel and 0.6% gel to an in vivo surrogate. It should be noted that one gel concentration does not serve all studies because different studies have different purposes and requirements.
It is interesting to note that Jilla et al. reported 0.2% as having the most realistic infusion pressure profile while Chen et al. reported 0.6% to be the most realistic. Jilla et al.1,7 compared the pressure profile of the in vitro gel to the in vivo data of the rat brain obtained by Prabhu et al.13 (0.5% was the closest concentration to 0.6% that Jilla et al. tested, therefore, hypothetically, if 0.6% agarose truly is indeed the best surrogate, one might expect 0.5% to be more realistic than 0.2%, however this was not the finding.7 Chen et al. proclamised of 0.6% as the most realistic phantom which was based on the in vivo work on the pig brain, not the rat brain. It is possible that the rat brain and pig brain produce significantly different infusion pressure profiles, making assigning one specific concentration as “most realistic” rather subjective. This is a crucial difference between the two studies and could partially explain the difference of opinion. Chen et al. did not test 0.2% gel. On the contrary, 0.6% is supported by the porosity analysis of Gillies et al., which was cited by Chen et al.1,12 Both concentrations have significant defense mounted in their favor; further work would be required to draw any conclusive statements about the “most realistic” agarose gel phantom.
The Agarose Model Today
Moving into the modern day, the focus of infusion studies involving agarose gels has expanded to include backflow, infusion protocols, and the effects of catheter design. Raghavan et al. showed backflow variability in 0.6% agarose gels, citing Chen et al. as a reference for the accuracy of the model. 3 They argue that the results of their work could be used by clinical software to determine backflow using the equations they developed, allowing backflow to be used as a boundary condition for brain infusions. Sillay et al. showed that using 0.2% agarose gels to compare two different catheter designs could be compared along with and their associated infusion protocols.14 In both cases, the results of gel studies can be applied to brain infusion equipment in healthcare, demonstrating the power that an accurate in vitro model can be used for. Low concentration agarose gels have more than proven their worth as a realistic in vitro model.
These expansions reflect increasing concern for patient safety during infusion-based treatment. Tissue damage from infusion pressure at the catheter tip can occur if the infusion rate is too high or if the catheter is too small.14 Tissue damage can also occur from catheter insertion, which can be further magnified by backflow along the catheter-tissue interface.14 Designing catheters and infusion protocols that prevent these issues from occurring or at least minimizing their effects is important for their use in a clinical setting. Sillay et al. tested a few of these designs, tracking infusion volumes and relating them to the amount of backflow observed and distribution of infusate through their agarose gel model (Figure 5).14 It was shown that backflow increases with increasing infusion rate and infusion volume, showing that infusion protocol can have a profound effect on tissue damage. It was determined that some catheters may be more effective at preventing backflow than others. Of the two catheters tested, the Engineering Resources Group (ERG) valve-tip catheter was likely more effective at reducing backflow (p = .0091). Design does make a difference in patient health and safety.15
Numerous other works also highlight the need for well-designed catheters and protocols. Casanova et al. reported that faster needle insertion speeds correlated with less tissue damage and less backflow in their 0.6% agarose hydrogel model.16 Results suggest that slower insertion speeds promote tissue damage, causing accumulation of tissue at the needle tip, creating a gap between the needle and the tissue, resulting in greater backflow. These findings were compared to in vivo brain tissue and a similar relationship was observed. Chen et al. details a method with which infusate distribution and concentration can be measured with MRI. In their study, the method was tested with a 1% agarose hydrogel.1 Such a system has the potential to provide a clinically relevant benchmark for numerous factors concerning infusion, especially target site selection and infusion rate. A deeper understanding of these variables will allow for safer and more effective protocol design. Sindhwani et al. published a method with similar goals tested in 0.6% agarose but uses optics and light intensity to measure distribution rather than MRI.17 Linninger et al. and Linninger et al. demonstrate the development and efficacy of a mathematical model based on transport phenomena that accurately predicts distribution for a given set of parameters that vary depending on the target site and the infusate.18,19 The computer simulations were verified with a 0.6% agarose gel. Linninger et al. used the simulations which were also verified in vivo in the rat brain. With further refinement, such a simulation could provide patient-specific treatment methods and protocols. Physicians would be able to view the infusion before it actually occurs, further refining the treatment for the patient. Yin et al. tested a stepped cannula design in a 0.25% agarose model and reported the backflow observed.20 Compared to a non-stepped cannula, the stepped cannula was proficient at limiting backflow and evening distribution, even at very high infusion rates and volumes. The term “stepped” refers to a funneling of the stent to a narrower tip. These findings were confirmed in rat striatum. This work is pertinent to the design of infusion equipment and targeting techniques. All of these findings contribute to making infusion a safer and more reliable treatment method.
Agarose gels are not without their limitations. Raghavan et al. explains that the theory they operationalised is dependent on the assumption of steady-state, which does not necessarily hold in reality.3 Also, they note that in vivo infusions tend to spread more quickly along the paths of blood vessels, skewing the normally spherical morphology of the infusate cloud, as shown in Figure 2. This factor is not present in a standard agarose gel. Sillay et al. describes numerous more limitations concerning the use of agarose gels.14 During catheter insertion, the integrity of the gel can be easily compromised or the catheter itself can rotate out of its intended position. In addition, large infusate (40 kDa) or highly charged infusate tends to diffuse through the gel much more slowly than that seen in in vivo studies using similar infusate. These factors can contribute to a significant loss of accuracy in the gel model. Regardless, for small infusate, Sillay et al. maintain confidence that low concentration agarose gels can potentially serve as accurate brain surrogates for infusion studies.14
Conclusion
In this work, the history of the use of agarose gel as an in vitro model for brain infusion studies and the benefits of doing so are briefly explored. It is evident that although agarose gels have significant limitations, the implications and effects they could have on healthcare of the future are substantial. Agarose gels provide an excellent platform for the study of in vitro model of the mammalian brain.
The article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of Interests: None, Source of funding: None.
Received Date: 13 February 2013; Revised Date: 12 April 2013; Accepted Date: 24 May 2013
References
1. Chen ZJ, Gillies GT, Broaddus WC, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. Journal of Neurosurgery. 2004; 101(2): 314–22.
2. Chen ZJ, Broaddus WC, Viswanathan RR, et al. Intraparenchymal drug delivery via positive-pressure infusion: Experimental and modeling studies of poroelasticity in brain phantom gels. Ieee Transactions on Biomedical Engineering. 2002; 49(2): 85–96.
3. Raghavan R, Mikaelian S, Brady M et al. Fluid infusions from catheters into elastic tissue: I. Azimuthally symmetric backflow in homogeneous media. Physics in Medicine and Biology. 2010; 55(1): 281–304.
4. Margolis S. Separation and size determination of human serum lipoproteins by agarose gel filtration. Journal of Lipid Research. 1967; 8(5): 501.
5. Arlinghaus RB, Kaczmarczyk W, Polatnick J. Electrophoretic characterization of foot-and-mouth disease virus-specific ribonucleic acid. J Virol. 1969; 4(5): 712–8.
6. Fujii T, Yano T, Kumagai H, et al. Scaling analysis of the concentration dependence on elasticity of agarose gel. Bioscience Biotechnology and Biochemistry. 2000; 64(8): 1618–1622.
7. Jilla YD, Gillies GT, Broaddus WC, et al. Univ. Virginia Sch. Eng. Appl. Sci., Charlottesville, Tech. Rep. UVA/640 419/MANE99/103, 1999.
8. Chen ZJ, Fillmore HL, Prabhu SS, et al. Univ. Virginia Sch. Eng. Appl. Sci., Charlottesville, Tech. Rep. UVA/640 419/MAE00/104, 2000.
9. Ahmed AJ, Gillies GT, Broaddus WC, et al. Catheter drag force measurements using a gel model of the brain. Univ. Virginia Sch. Eng. Appl. Sci., Charlottesville, Tech. Rep. UVA/640 419/MAE00/102, 2000.
10. Howard MA, Abkes BA, Ollendieck MC, et al. Measurement of the force required to move a neurosurgical probe through in vivo human brain tissue. Ieee Transactions on Biomedical Engineering. 1999; 46(7):891-894.
11. Gillies GT, Allison SW, Tissue BM. Positive pressure infusion of fluorescent n anoparticles as a probe of the structure of brain phantom gelatins. Nanotechnology. 2002; 13(4): 484–6.
12. Gillies GT, Wilhelm TD, Humphrey JAC, et al. A spinal cord surrogate with nanoscale porosity for in vitro simulations of restorative neurosurgical techniques. Nanotechnology. 2002; 13(5): 587–91.
13. Prabhu SS, Broaddus WC, Gillies GT, et al. Distribution of macromolecular dyes in brain using positive pressure infusion: A model for direct controlled delivery of therapeutic agents. Surgical Neurology. 1998; 50(4): 367–75.
14. Sillay K, Schomberg D, Hinchman A, et al. Benchmarking the ERG valve tip and MRI Interventions Smart Flow neurocatheter convection-enhanced delivery system’s performance in a gel model of the brain: employing infusion protocols proposed for gene therapy for Parkinson’s disease. Journal of Neural Engineering. 2012; 9(2): 026009.
15. White E, Bienemann A, Malone J, et al. An evaluation of the relationships between catheter design and tissue mechanics in achieving high-flow convection-enhanced deliv ery. Journal of Neuroscience Methods. 2011; 199(1): 87–97.
16. Casanova F, Carney PR, Sarntinoranont M. Influence of Needle Insertion Speed on Backflow for Convection-Enhanced Delivery. Journal of Biomechanical Engineering-Transactions of the Asme. 2012; 134(4): 8.
17. Sindhwani N, Ivanchenko O, Lueshen E, et al. Methods for Determining Agent Concentration Profiles in Agarose Gel During Convection-Enhanced Delivery. Ieee Transactions on Biomedical Engineering. 2011; 58(3): 626–32.
18. Linninger AA, Somayaji MR, Mekarski M, et al. Prediction of convection-enhanced drug delivery to the human brain. Journal of Theoretical Biology. 2008; 250(1): 125–38.
19. Linninger AA, Somayaji MR, Zhang LB, et al. Rigorous mathematical modeling techniques for optimal delivery of macromolecules to the brain. Ieee Transactions on Biomedical Engineering. 2008; 55(9): 2303–13.
20. Yin D, Forsayeth J, Bankiewicz KS. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. Journal of Neuroscience Methods. 2010; 187(1): 46–51.