
Annals of Neurosciences, Volume 18, Issue 2 (April), 2011
Behavioral analysis after sciatic nerve compression in albino rats
KEY WORDS
Sciatic nerve
Behavioral tests
Locomotor behavior scoring technique
Run way
Corresponding Author:
D. C. Mathangi, Ph. D.
Tel : +91 9940635874
E-mail : mathangidc@hotmail.com
ABSTRACT
Background: Walking track analysis which has been widely used to examine the recovery of gait functions in rats with sciatic nerve injury. Purpose: The present study was aimed to objectively analyze and quantify the degree of functional recovery in locomotor behavior of rats after inflicting sciatic nerve crush injury. Methods: Wistar rats trained on various runways, viz., narrow beam, grid and staircase, were subsequently tested following sciatic nerve crush injury. Results: Locomotor ability of injured rats on runways gradually recovered to the level that was not significantly different from their corresponding preoperative level by the sixth postoperative week. Conclusion: Conventional run ways can be objectively used for quantification of the level of recovery.
doi : 10.5214/ans.0972.7531.1118203
Introduction
Sciatic nerve (SCN) is the largest in body. Following the transection or compression of SCN, the paralysis of all muscles below the knee with foot drop, the dragging of ipsilateral toes in the affected foot while walking or inability to stand on toes is noticed. The SCN model has, therefore, been extensively used to examine the recovery of motor as well as sensory functions in peripheral nerve regeneration research.1–5
A variety of traditional methods, viz., muscle electrophysiology, measurements of axonal transport6 and functional tests, viz. the Tinel’s sign7 and the grasping test8 are used to evaluate the recovery of functions after SCN injury in animals. It has been proved that SCN has an equivalent capacity for regeneration in rats and subhuman primates.1 Investigators have widely applied the walking track analysis as a reliable method for the assessment of motor functions after nerve injury. In SCN research, De Medinacelli et al first designed a quantitative method of analyzing the nerve functions in rats known as “Sciatic function index” (SFI), based on the measurements of footprints made on X-ray film.9 Later this method was modified2,3-10, 11 using video recording of the foot-impressions of SCN injured rats. In subsequent investigations, the footprint technique used in albino rats were modified to examine the functional recovery of tibial and peroneal nerves after injury in mouse.12 Researchers have applied static footprint analysis to evaluate the sciatic nerve functions in rat locomotion.13, 4 Nevertheless, the quantitative analysis of nerve functions through footprint measurements can be compromised by the variations in print length parameters with gait velocity14 or smearing of prints due to the dragging of tail.15
In neuroscience research, the effects of lesion have also been correlated with recovery of behavioral functions in laboratory animals. For example, McBride and colleagues16 assessed the ability of striatum lesioned rats to use their forelimbs and forepaws with the cylinder test. Similarly, some groups of researchers have used a battery of sensitive behavioral tests and scoring techniques for the quantitative evaluation of motor functions following the spinal cord injury in rats,17–19 cats20 and in monkeys.21, 22
On perusal of the available literature in peripheral nerve injury research, it is conspicuous that the assessment of functional recovery in motor behavior is mostly conducted by the measurements of footprint variables in animals. Simple and complex tests which are widely used in brain and spinal cord injury research have not been used to assess the recovery of motor functions after SCN injury in rats. Hence, we proposed to assess the functional recovery following SCN injury in rats by simple and complex locomotor tests using a locomotor behavior scoring technique.
Methods
The approval from the Animal care and Institutional Ethical Committee of University of Madras was obtained prior to this study. Adult Wistar strain albino rats (n=6) weighing 200–250 gm were used. They were housed individually in polypropylene cages (29cm X 22cm X 14cm) in a temperature and humidity controlled room with 12:12h light/dark cycles. Animals were fed with rat pellets (Gold Mohur, India) and water ad libitum. Rats were gently trained to perform the locomotion on a series of simple and complex runways prior to surgery. At the end of training period, the ability of animals on different behavioral tasks were tested for three additional days preoperatively by two independent observers other than the trainer so as to ensure a consistent evaluation of animal’s maximum ability.
Previously existing locomotor behavioral tests and scoring techniques, designed to study the recovery of motor functions after spinal injury in rats,17–19 was modified for detailed examination of the functional status and progressive modifications in different components of locomotion after SCN injury in rats. The animals were subjected to behavioral testing both before and after crushing the SCN for analyzing the alterations of locomotor functions at weekly intervals, under the evaluation of locomotor behavior scoring technique. (Table 1)
Locomotor Testing
Gross motor score: The walking pattern and spontaneous activity of rats were observed in a large open field at ground level. The functional status of hindlimb movements during locomotion was assessed at weekly intervals post operatively (Fig 1A).
Table 1: Evaluation of locomotor behavior of rats on different runways
| For gross motor score | |
|---|---|
| Score | Behavior |
| 0 points | No movement in hindlimb, no weight bearing |
| 2 points | Mild movement in hindlimb, no weight bearing |
| 4 points | Frequent and/ or vigorous movement of hindlimb, no weight bearing |
| 6 points | Hindlimb can support weight partially and weak attempt in walk |
| 8 points | Walking with only mild deficit |
| 10 points | Normal walking (near normal) |
| For wide runwayFor wide runway | |
| Score | Behavior |
| 0 points | No movement in hindlimb, no attempt to walk and weight bearing |
| 1 points | Weak and delayed attempt to walk with significant change in steps and time taken to cross the runway |
| 3 points | Good attempt to walk with significant change in time taken to ross the runway |
| 5 points | Good attempt to walk without any significant change in steps and time taken to cross the runway |
| For narrow beam runway, grid runway and staircase runway | |
| Score | Behavior |
| 0 points | No attempt to walk, no weight bearing |
| 2 points | Weak and/or delayed attempt to walk in the runway (initiated one or two steps but could not complete the task |
| 4 points | Good attempt to support body weight, weak attempt to walk in the runway with frequent errors seen |
| 6 points | Good attempt to walk in the runway with significance in steps and time taken to cross the runway |
| 8 points | Good attempt to walk in the runway with only mild deficits, and no significant change in time taken to cross the runway |
| 10 points | Good attempt to walk in the runway, no significant change in time compared to control animals |
| For inclined plane test | |
| Score | Behavior |
| 0 points | No attempt to stand and walk with hindlimb |
| 2 points | Weak and /or delayed attempt to walk (initiated one or two steps and could not complete the task) |
| 4 points | Good attempt to support body weight, weak attempt to walk in the runway |
| 6 points | Good attempt to walk with few foot slips and significant changes in steps and time taken while crossing the runway |
| 8 points | Good attempt to walk in the runway with mild deficits and no significant change in time taken to cross the runway |
| 10 points | Good attempt to walk in the runway without significant change in steps and time taken to cross runway as similar to normal animals |
| For swimming test | |
| Score | Behavior |
| 0 points | No movements in operated hindlimb, no attempt to swim |
| 1 points | Weak and delayed attempt to swim with significant change in time taken to reach the platform |
| 3 points | Good attempt to swim with significant change in time taken to reach the platform |
| 5 points | Good attempt to swim without significant change time taken to reach the platform |
| For climbing test | |
| Score | Behavior |
| 0 points | No attempt to climb up the platform with its operated hindlimb |
| 2 points | Mild attempt to climb up the platform with its operated hindlimb |
| 4 points | Weak attempt to climb up the platform with slips and significant change in time |
| 6 points | Good attempt to climb up the platform with slips and significant change in time |
| 8 points | Good attempt to climb up the platform without any slips with significant change in time |
| 10 points | Good attempt to climb up the platform with the performance near to normal animals |
Grid runway: The grid (5cm X 5cm holes) of 180 cm length was used in this test. The accuracy of limb position and its precise motor control was assessed with grid runway. Rats were trained to walk and cross the runway in both directions. The locomotor ability of animals was observed by counting the number of steps and time taken to cross this runway. The progressive modifications in the locomotor behavior were assessed (Fig 1B).
Inclined plane runway: Rats were trained to walk on a wooden board fitted at an angle of 45 degree. The ability of animals to maintain and balance its body position during locomotion on inclined plane was assessed as mentioned above (Fig 1C).
Wide runway: In this test, a flat wooden board (180cm X 15cm) was kept at one meter above the ground level. The ability of rats to walk over the grid runway was assessed as mentioned above (Fig 1D).
Narrow beam runway: A narrow wooden beam (180cm X 2.5cm) was used in this test. The animals were trained to walk in both directions on the beam. Performance of rats to cross this runway was assessed as similar to the wide runway (Fig 1E).
Staircase runway: Rats were trained to ascend and descend a staircase. The locomotor performance of animals was assessed by counting the number of steps and time taken to climb up and down the runway (Fig 1F).
Swimming test: A tank filled with water to a depth of 30 cm was used in this test. A steel mesh ladder was hung on one side of the tank, as a platform. The ability of rat to swim the length of the tank and climb out of water to reach the platform was assessed (Fig 1G).
Climbing test: Rats were trained to climb onto a platform for a food reward. The performance of animal’s hindlimb and its ability in terms of maximum time (in seconds) to climb up the platform was noted.

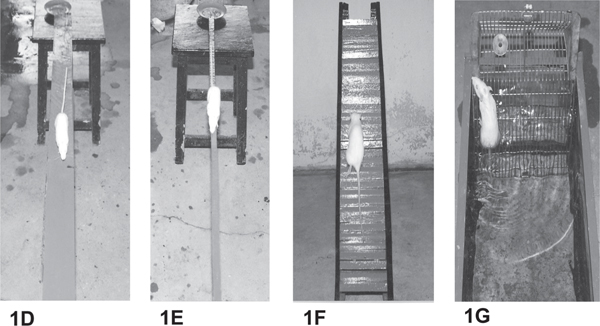
Fig. 1: Photographs showing the locomotor behavior of trained rats on different runways. (A) Gross motor score, (B) grid runway, (C) inclined plane runway, (D) wide runway, (E) narrow beam runway, (F) staircase runway, and (G) swimming
Sciatic Nerve Crush: After the completion of training and pre¬surgical assessment, the rats were anaesthetized with ketamine (80 mg/kg, IP) and xylazine (10 mg/kg) cocktail. The anesthetized animal was placed in a sterile surgical table. Body temperature was maintained in these animals during surgery using a heating pad. The surgical area was shaved carefully with a sterile razor and disinfected with betadine solutions. Under a dissecting microscope, a 2-3 cm dorsal longitudinal incision was made slightly to the left of midline over the pelvic girdle in the right hindlimb. Then a 10 mm deep incision was made for separation of muscles and left proximal SCN was exposed in mid-thigh region.23 In exposed SCN, a 3 mm segment was gently crushed for duration of 15 minutes by using a sterilized artery forceps, applied perpendicular to the SCN (Fig 2 A, B). The crushed site of SCN was flattened, but the nerve continuity was not completely interrupted. Later, the muscles and skin were sutured in layers to cover the incised area in the thigh region. Necessary measures were taken to minimize the loss of blood and pain in animals during the surgery. Operated animals received subcutaneous injection of saline to compensate for blood and general body fluid loss, if required. Following recovery from anesthesia, animals were returned to their home cages for post operative surgical care and then allowed to recover for one week. Antibiotics (Gentamicin 10 mg/day,i.m.) and analgesic (Pentazocine 0.5 mg/kg,i.m.) were administered daily for 4 days following surgery. The sutured area was gently cleaned daily and betadine cream was applied to avoid the infection. At the end of recovery period (1 week after SCN crushing), the animals were again subjected to locomotor testing for the weekly postoperative observations for a period of 6 weeks.
Statistical Analysis
The data obtained was analyzed by One way Analysis of Variance (ANOVA) and, if the F test ratio was significant, Newman Keul’s multiple comparison was performed with the level of significance at P < 0.05.
Results
Immediately after mild SCN crush injury, a complete paralysis of the operative foot was observed. Hence, the rats were left undisturbed for 1 postoperative week as this was considered to be necessary for recovery from the surgical trauma. From the 2nd postoperative week onwards, all SCN crushed rats were retested for progressive functional recovery of their reflexes responses and locomotor behavior on runways. No mortality or serious infections were noted in these animals.
Locomotor testing
Gross motor score: In this test, the locomotor functions of SCN crushed animals were difficult to quantify accurately similar to the other behavioral tests. The animals with SCN crush injury showed a mild movement in the ipsilateral side of hindlimb by the end of 2nd postoperative week (4 points). By the 4th postoperative week, walking and climbing pattern of operated animals quickly improved and recovered to near normal animals. However, on careful examination, we observed a mild deficit in unfolding of toes of their hindlimbs while walking on the ground (8 points). In the subsequent weeks, there was no further improvement in the performance of SCN crushed animals in gross motor score till the end of observation period.
Runways: SCN crushed rats could not successfully complete the task of walking on different runways during initial part of postoperative period. This inability to walk was typically prolonged in narrow beam, inclined plane, and staircase runway. Even though all animals showed improvement in their ability to traverse the wide runway as early as 2nd postoperative week (1 point), such improvement was seen only by the 3rd week on the other runways. The operated animals gained confidence to cross (with a few errors) the narrow beam (4 points) and grid runway (4 points), climb up/down in inclined plane (6 points) at the end of 3rd postoperative week and staircase runway (4 points) at the end of 4th postoperative week. In the subsequent observation period, all SCN crushed animals showed improvement in the walking performance of crossing different runways quickly and completed the task without any significant difference from their corresponding preoperative value by the 4th postoperative week (5 points) for wide runway, 5th postoperative week (8 points) for grid, narrow beam (8 points), inclined plane runway (8 points) and 6th postoperative week (8 points) for staircase runway respectively. (Fig 3 A, B, C, D, E, F)
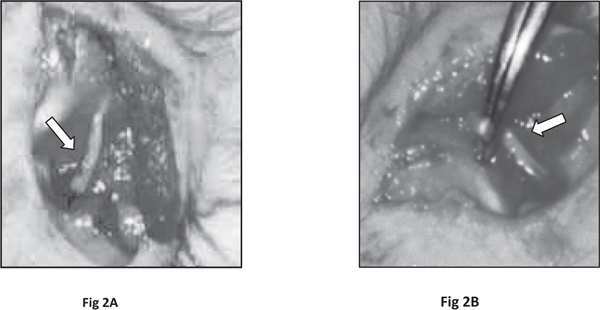
Fig. 2: Photographs illustrating the exposure of sciatic nerve (A) and crush injury induced in the exposed sciatic nerve using the artery forceps (B). An arrow indicates the exposed sciatic nerve
Climbing and Swimming: All the SCN crushed animals showed weak and delayed attempt in swimming (1 point) and climbing behavior (4 points) at the end of 2nd postoperative week. However, the performance of operated animals improved progressively in this tests in subsequent period and attained to near preoperative value by the end of 3rd postoperative week for swimming (5 points) and climbing (8 points). (Fig 3 F)
Discussion
In the present study, we have applied a series of simple and complex tests for estimating the recovery of gait after mild SCN crush injury in rats, using locomotor behavior scoring technique. Despite recent advances in microsurgical techniques, the understanding of functional recovery following repair of transected peripheral nerve remains poor. Even though traditional methods such as histomorphometry and electrophysiology are universally employed in neural regeneration research for assessment of nerve functions after injury, it does not satisfactorily correlate with behavioral recovery. For example, withdrawal response after application of a small current on sole of foot is regarded as an easy and simple method, but can only assess return of sensory functions after peripheral nerve damage.24 Using axon count and degree of myelination, it is imprecise to study the mechanism of axonal regeneration towards appropriate target organ.25
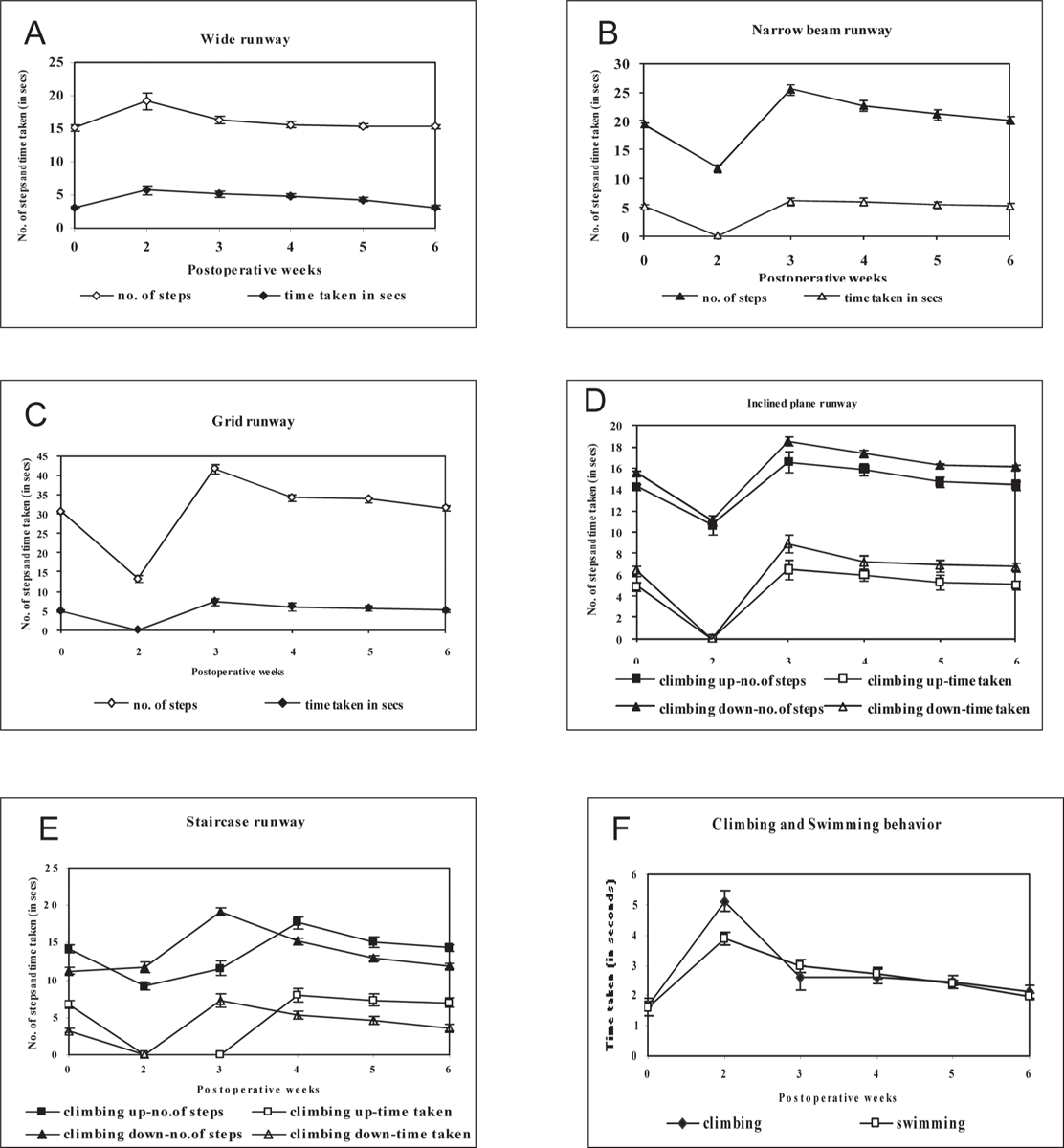
Fig. 3: Illustrations of the time course for the recovery of locomotor function of sciatic nerve crushed rats on different runways (A) Wide runway, (B), Narrow beam runway, (C) Grid runway, (D) Inclined plane runway, (E) Staircase runway, (F) climbing and swimming
Most investigators of peripheral nerve injury research have widely employed the footprint analysis as a common tool to assess the SCN functions. In 1982, De Medinacelli et al9 employed the SFI for the analysis of SCN functions in rats; but the validity of SFI is unsatisfactory and not accepted universally. Further, some scientists suggested that the methodology of footprint measurements with SFI followed in past investigations still remains replete with many controversies and some of which are listed as follows: (a) it is important to observe the rat’s progress along the locomotor runway; when the animal is placed for first time in the corridor, it often stops pressing the foot pad and heel down creating a false, untypical long print length,14 (b) the print length parameters was varied with gait velocity,3 (c) it is difficult to obtain clear print marks owing to contractures, autotomy, smearing of the print, dragging of the tail or contamination with foot prints,15 (d) several attempts are required to obtain the most representative prints for analysis.26 Hence, there is also a need to use the behavioral methods for analysis depending on the nature of motor deficits and to define which behavioral capacities return or fail to recover after nerve injury.
Despite the use of morphometric and electrophysiological techniques in peripheral nerve regeneration research, behavioral analysis also forms an ultimate assay to study the different features of nerve functions. In the present study, we used a series of behavioral tests under the evaluation of locomotor scoring technique that can provide additional details on the recovery of motor functions and to understand the capacity of regeneration of injured peripheral nerve. Researchers in this area have suggested the importance of applying the behavioral tests for the analysis of recovery of motor functions in animals, because (1) the use of behavioral analysis helps specify the affected part from the undamaged indicating the masked deficits. (2) it allows quantitative analysis of the nature of deficits, which could be useful in identifying the different kinds of abnormalities/injuries. (3) the use of multiple behavioral tests minimizes the possibility whether experimental artifact in any single testing procedure will significantly alter the results.17,18,27
Behavioral test such as the Tarlov Scale,28 also termed as gross motor score, is a major component of behavioral assessment and involves a passive, subjective evaluation of the animal’s hindlimbs, without any examination of reflex function. In the present study all SCN crushed animals recovered to near normal behavior in this test by the 4th postoperative week and the points contributed by the motor score did not exceed of the total points assigned. However, the performance of operated animals in motor score do not provide adequate information for subsequent treatments (namely drugs, transplants) since they cannot control for altered motivational levels or reluctance to use an impaired limb.17 Since the use of behavioral outcomes suggest that different tests may be sensitive for various aspects of the recovery process, the combination of results from the entire protocol allows us to obtain the precise assessment of complete functional deficit than with any one individual test. For instance, although the SCN crushed rats crossed the wide runway similar to normal animals by the 4th postoperative week, the locomotor ability of these animals to complete the task successfully on grid and staircase runway took a longer duration and recovered to the near preoperative level by the 5th and 6th postoperative week respectively.
The rate of functional recovery and morphological regeneration of crushed nerves is directly proportional to the application of impact force. For example, using the SFI index, a few investigators have reported that recovery of motor functions in SCN crushed rats was found to attain the near presurgical level by the 14 and 39 postoperative day after 100 gm and 500 gm crush respectively.29 Sarikcioglu et al. also induced a severe crush injury in SCN of rats with Yasargil aneurysm clip and provided the evidence that nerve regeneration or recovery depends on trauma durations in which a longer recovery is needed after longtime pressure.30 In the present study, functional recovery of locomotor behavior after the induction of a mild SCN crush injury using the artery forceps in rats has reached the near preoperative level by the 6th postoperative week. Thus, it can be concluded that a strong correlation exists between the degree of nerve damage and subsequent recovery.
This preliminary report highlights the use of locomotor behavior as functionally objective tool in the assessment of recovery following SCN crush injury, however, a detailed research with correlation of functional recovery with histology and biochemical marker is warranted.
The article complies with International Committee of Medical Journal Editor’s uniform requirements for the manuscripts.
Competing interests – None, Source of Funding – None
Received Date : 22 January 2011; Revised Date: 28 February 2011
Accepted Date : 31 March 2011
References
1. Mackinnon SE, Hudson AR and Hunter DA. Histologic assessment of nerve regeneration in the rat. Plastic Reconstr Surg 1985; 75: 384–388.
2. Bain JR, Mackinnon SE and Hunter DA. Functional evaluation of complete sciatic peroneal and posterior tibial nerve lesions in the rat. Plastic Reconstr Surg 1989; 83: 129–136.
3. Walker JL, Resig P, Sisken BF, et al. Improved foot print analysis using video recording injury to the rat sciatic nerve. Restor Neural Neurosci 1994; 6: 189–193.
4. Varejao ASP, Cabrita AM, Geuna A, et al. Toe out angle: a functional index for the evaluation of sciatic nerve recovery in the rat model. Exp Neurol 2003; 183: 695–699.
5. Smit X, van Neck JW, Ebeli MJ, et al. Static footprint analysis: A time-saving functional evaluation of nerve repair in rats. Scand J Plastic Reconstr Surg Hand Surg 2004; 38: 321–325.
6. Tessler A, Gambetti LA and Gambetti P. Axonal growth during regeneration; a quantitative autoradiographic study. J Cell Biol 1980; 60: 1284–1335.
7. Lardi A, Copeland SA. Value of the Tinel sign in brachial plexux lesions. Annu Rev Surg (Engl) 1979; 61: 470–471.
8. Bertelli JA, Mira JC. The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J Neurosci Methods 1995; 58: 151–155.
9. De Medinacelli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 1982; 77: 634–643.
10. Carlton JM, Goldberg N. Quantitating integrated muscle function following reinnervation. Surg Forum 1986; 37: 611.
11. Lin FM, Pan YC, Hom C, et al. Ankle stance angle: A functional index for the evaluation of sciatic nerve recovery after complete transaction. J Reconstr Microsurgery 1996; 12: 173–177.
12. Inserra MM, Bloch DA and Terris DJ. Functional indices for sciatic peroneal and posterior tibial nerve lesions in the mouse. Microsurgery 1988; 18: 119.
13. Berver M. Video analysis of standing-an alternative analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods 2000; 102: 109–116.
14. Saraf M. Memory – mechanisms, tools and aids. Annals of Neurosciences 2009; 16(3): 119–122.
15. Meek MF, Den Dunnen WFA, Barlets HL, et al. Peripheral nerve regeneration and functional nerve recovery after reconstruction with a thin walled biodegradable poly (DL-lactide-3-caprolactone) nerve guides. Cell Materials 1997; 7: 53–62.
16. McBride JL, Behrstock SP, Chen EY, et al. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comp Neurol 2004; 475: 211–219.
17. Gale K, Kerasidis H and Wrathall JR. Spinal cord contusion in rat: Behavioral analysis of functional neurologic impairment. Exp Neurol 1985; 88: 123–134.
18. KS Lashley. Basic neural mechanisms in behaviour. Annals of Neurosciences 2010; 17(1): 28–30.
19. Kunkel-Bagden, Dai HN and Bregman BS. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp Neurol 1993; 119: 153–164.
20. Goto T, Hoshino Y. Electrophysiological, histological and behavioral studies in a cat with acute compression of the spinal cord. J Orthopaedic Sci 2001; 6: 59–67.
21. Suresh Babu R, Muthusamy R and Namasivayam A. Behavioral assessment of functional recovery after spinal cord hemisection in Bonnet monkeys (Macaca radiata). J Neurol Sci 2000; 178: 136–152.
22. Cain JH, Baggott C, Tilghman JI, et al. Recent Developments in the Study of Spinal Cord Injury and Neuropathic Pain. Annals of Neurosciences 2007; 14(4): 96–107.
23. Attal N, Filliatreau G, Perrot S, et al. Behavioral pain-related disorders and contribution of the saphenous nerve in crush and chronic constriction injury of the rat sciatic nerve. Pain 1994; 59: 310–312.
24. De Koning P, Brakkee JH and Gisper WH. Methods for producing a reproducible crush in the sciatic and tibial nerve of the rat and rapid precise testing of rectum of sensory function. Beneficial effects of melacrotins. J Neurol Sci 1992; 74: 237–246.
25. Mackinnon SE, Dellon AL and Brien JP. Changes in nerve fiber number distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 1991; 14: 116–122.
26. Hare GMT, Evans PJ, Mackinnon SE, et al. Walking track analysis: a long term assessment of peripheral nerve recovery. Plastic Reconstr Surg 1992; 89: 251–258.
27. Goldberger ME, Bregman BS, Vierck CJ. Jr., et al. Criteria for assessing recovery of function after spinal cord injury: Behavioral methods. Exp Neurol 1990; 107: 113–117.
28. Tarlov IM. Spinal cord compression studies III. Time limits for recovery after gradual compression in dogs. Arch Neurol Psy 1954; 71: 588–597.
29. Chen A. The functional recovery of peripheral nerve following defined acute crush injuries. J Orthopaedic Res 1992; 10: 657–664.
30. Sarikcioglu L, Yaba A, Tanriover G, et al. Effect of severe crush injury on axonal regeneration: A functional and ultrastructural study. J Reconstr Microsurg 2007; 23: 143–150.