Annals of Neurosciences, Volume 21, Issue 1 (January), 2014
Sonic hedgehog signalling pathway: a complex network
ABSTRACT |
Sonic Hedgehog (Shh) signalling cascade is one of the intricate signal transduction mechanisms that govern the precisely regulated developmental processes of multicellular organisms. Along with establishing the patterns of cellular differentiation to direct complex organ formation, it also has an important role in post-embryonic tissue regeneration and repair processes. Especially, Shh signalling is implicated in the induction of multifarious neuronal populations in central nervous system. There is compelling evidence of the involvement of Shh protein in the signalling network that regulates various morphogenetic processes such as the exquisite neural tube pattern formation. In the morphogenetic field, the activation of Shh signalling processes is intricately linked to the alterations at the molecular level in the structure of Shh protein that leads to its altered biophysical and biochemical reactivity. This brief article gives an overview of such complex cascade of events in Shh signalling and its transduction pathways. KEYWORDS: Sonic hedgehog, Holoprosencephaly, Notochord, Neural tube pattern, Autoproteolysis, Lipidation Corresponding Author: Nusrat J Mobassarah, Address: Institute of Integrated Cell-Material Science, Kyoto University, Yoshida Ushinomiyacho, Sakyo – ku, JAPAN, 606-8501, Tel: 81-80-757539869, Fax: 81-80-757539872, Email: snusratjahan@icems.kyoto-u.ac.jp doi : 10.5214/ans.0972.7531.210109 |
Introduction
Sonic Hedgehog (Shh) signalling pathway is one of the major trafficking networks that regulates the key events during developmental processes i.e growth and patterning of multicellular embryos.1 Aberration in the regulation and transduction of the Shh signalling pathway has been implicated in birth defects, tissue regeneration, stem cell renewal and cancer growth.2 The regulatory action of Shh signalling pathway is precisely linked to the secretion, uptake and translocation of ‘Shh protein’, an important Hedgehog (Hh) ligand precursors.3 Most importantly, Shh is one of the putative signalling molecules, which is implicated in the regulation of central nervous system (CNS) polarity and neural patterning.4 Named after “Sonic the Hedgehog”, a computer game played by children in the early 90s, ‘Sonic’ appeared to have two closely set eyes with a common scleral rim, this form suggest holoprosencephaly, a condition seen in murine null mutation of Shh gene (Shh ¯/¯).5 It is initially secreted in the neural tube by the notochord and believed to induce differentiation of the floor plate cells, motor neurons, and inter-neurons.6,7 The floor plate expresses Shh in response to the notochordal Shh signal.8 Gain and loss of function studies have revealed that Shh is not only necessary for ventral cell type induction but it has also been observed that targeted Shh gene disruption in mouse produces cyclopia when neural tube cells are absent and the recessive mutation results in a severe holoprosencephaly.9 In humans, Shh mutations are heterozygous and result in a variable clinical expression of holoprosencephalic phenotype.7 We have briefly reviewed the complex networks of the Shh signalling pathway with a particular emphasis on the molecular level alterations in Shh and its transduction resulting midbrain architectural pattern formation.
Shh graded signal inducing ventralization
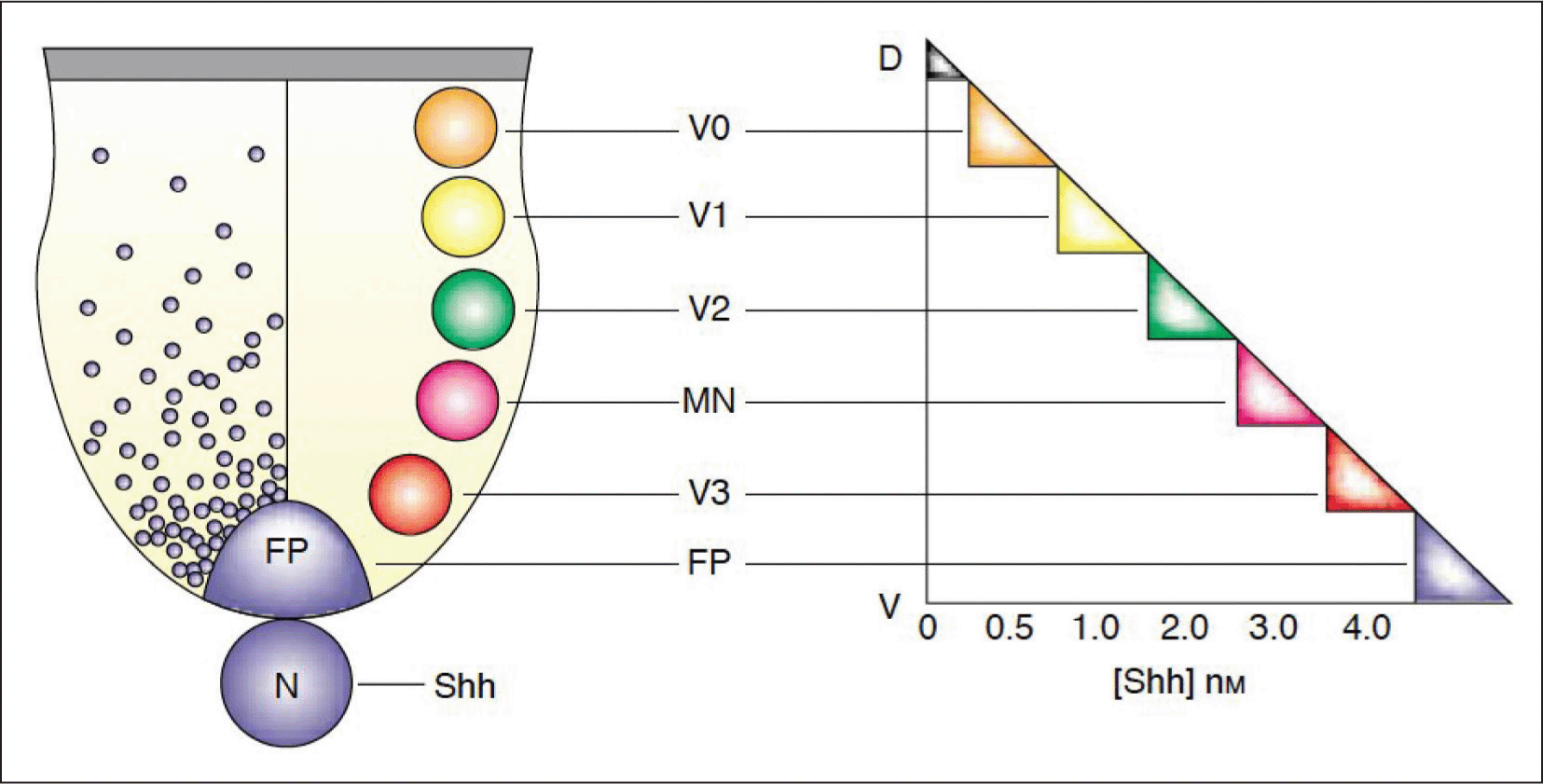
Shh is one of the three vertebrate hedgehog genes (Indian, Desert, and Sonic) homologous to the Drosophila hedgehog.5 Shh acts as a morphogen, and induces different cells fates at different concentration thresholds, low concentrations induce ventral neurons, high induce motor neurons and very high floor plate cells. In Shh naïve neural plate explants, exposure to different concentration of Shh protein resulted in different cells.11,12 During the spinal chord formation, Shh is generated ventrally in the notochord and floor plate cells inducing several classes of ventral interneuron progenitors (V0-V3) (Fig. 1).12 It also helps to specify the identity of motor neuron progenitors and results in five classes of neurons.12 Inhibition of Shh signalling stops differentiation.13,14

Fig. 1: Schematically represented alteration of ventral neuronal fate induced by different concentrations of Shh protein.12 D, dorsal neural tube; V, ventral neural tube; MN, motor neurons, FP, floor plates; Shh, Sonic Hedgehog. (Reproduced with permissions from Reference 12).
High Shh concentration gradients in ventral spinal cord have also been found to activate class II genes such as NKx2.2 and Pax6, which encode for homeodomain transcription factors, bringing about the effect of the Shh gradient.12 On the other hand, it is found to inhibit class I genes. Thus Shh is instrumental in conserving ventral identity of the neural plate and establishing specific progenitor cell domains.15 However, in Drosophila, Shh functions both locally and over short (8-10 cell) distances.16
The core of Shh signalling pathway and Shh auto modification
As described above, the core of the diverse spatio-temporal responses of Shh signalling pathway is based on Shh protein gradient, which is likely to be generated by lipid modification of membrane anchored Shh and its movement in the morphogenetic field. Shh is initially synthesized as a 45-kDa precursor, it is auto proteolytically cleaved into two secreted peptides: a 19 kDa (amino terminus) Shh-N and 26 kDa (carboxy terminus) Shh-C. Mutations that block this auto proteolysis impair Hh function.17,18 Shh-N has been shown to mediate signalling in vertebrates and invertebrates while Shh-C is believed to mediate the autoproteolysis reaction.19 Auto-proteolysis results in the addition of a cholesterol moiety at the C-terminus of Shh-N. After this addition, the Shh-C catalytic portion diffuses away and a palmitoyl group is added to the N-terminal of Shh (Fig. 2).20 Palmitoylation of Shh-N is catalyzed by an acyltransferase, the Skinny hedgehog (Ski) acyltransferase.21,22 Cholesterol addition is significant in the secretory regulation and long-range activity of the Shh protein.23,24 Similarly, palmitoylation increases the inductive potency of Shh.25 Studies carried out in vitro and in knockout mice reported previously, suggest that palmitoylation of Shh protein is hindered in absence of cholesterylation implicating that dual lipid modification (Fig. 2) is a key factor required for long range Shh signalling.26
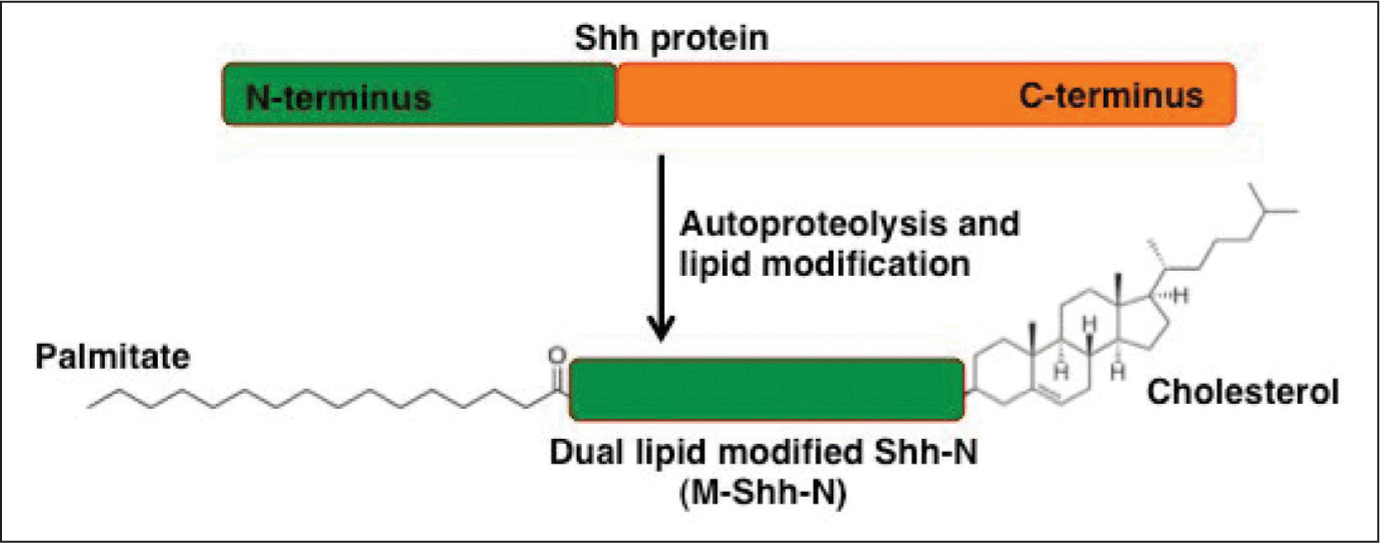
Fig. 2: Schematic representation of dual lipid modification of Shh protein.
Shh transport system
Secretion and distribution
The cholesterol-modified Hh is tethered to the cell membrane. Its transport is a highly complex process, which is regulated at different levels. Successful transportation of Shh from the secretory cell involves a series of steps. Firstly, Shh-N is “multimerized” to become dual lipid-modified Shh (M-Shh-N), which makes Shh soluble and diffusible enabling long range signalling.24 Secondly, it has also been suggested that “Dispatched” (Dsp), a 12-pass transmembrane transporter protein with a sterol sensing domain (SSD) interacts with the cholesterol modified Shh-N, and releases it from its tether to the plasma membrane of secretory cells enabling it for long range signalling.27 Thirdly, Heparin sulphate proteoglycans (HPSG), such as Dally-like (Dlp) and Dally are responsible for extra-cellular transport of the Hh.27 HPSG are believed to transport M-Shh-N to its receptor Patched (Ptc) allowing M-Shh-N to move from one cell to another. Finally, an enzyme “Tout-velu” regulates the movement of M-Shh-N. “Tout-velu” works indirectly as it is needed in the heparin sulphate biosynthesis.28
Activation of Shh signalling
The activation of Shh signal requires binding of Shh to the Ptc mediated Smoothened (Smo) (Ptc-smo) receptor complex and induction of downstream signalling cascade. This is a heterodimeric receptor complex. Ptc-Smo heterodimeric receptor complex consists of two trans-membrane subunits, namely Ptc and Smo. Ptc gene codes for a 1286 amino acid protein having at least seven putative transmembrane α helices, which plays a major role in the down stream Shh signalling. In Drosophila, Ptc has been shown to be integral for correct patterning of segments, devoid of which all cells attain segment border cell characteristics.29 Binding studies using labelled Shh have shown that Hh receptor is encoded by Ptc.30 Ptc contains a sterol-sensing domain (SSD), which interacts with the cholesterol modified Shh.27 Binding of Shh signalling protein to Ptc regulates activity of Smo. Shh free Ptc has been found to act sub-stoichiometrically to suppress Smo activity, and thus it is critical in specifying the level of signalling activity.31
The Ptc suppressor normally functions as a transmembrane molecular transporter. Ptc is also believed to indirectly inhibit Smo activity, possibly through changes in distribution or concentration of a small inhibitor molecule.32 Smo is a member of the Frizzled (Fz) family of seven-pass trans-membrane receptors.33 As a response to the binding of Shh with Ptc, Smo is activated and stabilized. The activated Smo initiates the Shh downstream signalling cascade by encoding membrane proteins, which are similar to G protein-coupled receptors. Smo is also believed to encode a receptor of the Shh signal.34 It generates intracellular signals that regulate several protein kinases, which activate a class of transcription factors known as cubitus interruptus (Ci) proteins and glioblastoma (Gli) proteins.35
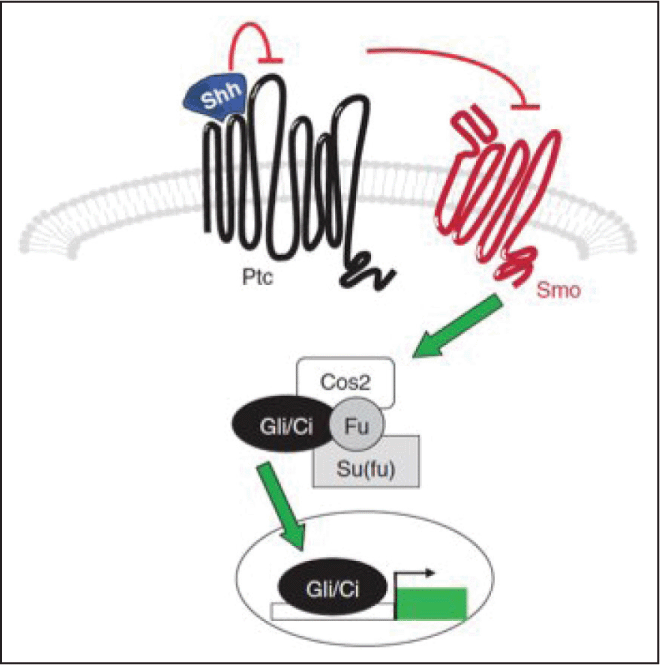
Fig. 3: The Sonic Hedghehog (Shh) signalling pathway: Activation of signalling pathway is connected to various transmembrane proteins (Ptc, Smo), transcription factors (Ci/Gli) and protein kinases (Su(fu), fu) (Reproduced with permissions from Reference 31).
SHH Transduction
Genetic studies have identified that transduction of the Hh-encoded signal is mediated by the activity of four segment polarity genes, Ptc, fused (Fu), costal-2 (Cos-2) and (Ci). Transcriptional activation of Hh target genes requires Ci, a 155 kDa cytoplasmic zinc finger protein (Ci155).36 Three Gli proteins Gli1, Gli2 and Gli3 are expressed in vertebrates, in overlapping domains and are partially redundant. Gli2 and Gli1 show activator functions that are dependent on Hh. Gli2 and Gli3 are proteolyzed to produce a repressor form, which is able to inhibit Hh expression. Hh regulates Gli3 repressor activity, while Gli2 is independent. This suggests uneven portioning of the separate activator and repressor functions of Ci among the three Glis, yielding proteins with related yet distinct properties.37 The three Gli proteins in vertebrates allow complex responses within target fields. The Hh signal cellular response depends on both level of ligand exposure and the individual Gli genes expressed.38,39 Cos2 is a kinesin like protein, which is associated to microtubule. Cos2 is mainly the motor domain that binds to ATP and microtubules and hydrolyses ATP.37,40 Fu is a segment polarity gene, which is phosphorylated in response to Hh signalling and involves an activation loop for Fu transcription.37,41 Fu binds to Cos2 by its carboxy-terminal and phosphorylate it.42 Suppressor of fused (Sufu), Human Sufu interacts with Gli1, Gli2, Gli3, and Slimb, it has been indicated as a negative regulator of the hedgehog-signalling network.43 Ci, Cos2, Fu, and Sufu form a tetrameric complex for the downstream signalling process. This complex results in transcription activity of Ci. In the absence of Hh signal, the tetrameric complex is attached to the microtubules in the cytoplasm by Cos2 component. Sufu attaches itself to Ci by adhering to the Sufu domain of Ci and Cos 2 attaches to the Ci by binding to the Ci-CORD domain.37,44 Cos2 is responsible for the proteolysis of Ci. This proteolytic reaction converts Ci from active Ci155 to inactive Ci75 state. The inactive Ci75 conversion requires phosphorylation of Ci by dPKA, CK1, and Sgg. Slimb, Sufu, and SAP18 are also implicated in this conversion. The inactive Ci75 when translocated to the nucleus fails to bring about transcription of target genes Ptch, Wg, and Dpp.37,38 Intermediate Hh stimulation dissociates the complex from the microtubules and induces inhibition of Ci cleavage and dephosphorylation of Ci. Ci is conserved in its Ci155 form; it binds to Sufu, restricts Ci155 nuclear translocation but attenuates its transcriptional activity. Higher Hh stimulation dissociates Sufu from Act-Ci155. Sufu and cofactor dCBP upregulate target genes Ptc, Wg, and Dpp.38,41 Ptc gene expresses Ptc protein, thus increases the transmembrane Ptc protein which attracts and attaches Shh and inhibits the Shh long range spread.37 Dpp acts as BMP2 and BMP4 at short ranges and it provides dorsal-ventral positional information to the embryonic ectoderm.
Conclusions
Shh is not only considered as a local short-range signalling molecule but it has also been implicated in regulating ventral patterning in the neural tube. It is perceived as an inductive factor acting at both short and long range and found to be involved in a range of processes and functions of vertebrates and invertebrates. These processes range from axial patterning, cell survival to cell proliferation and differentiation. Genetic studies investigating Shh have explained many integral aspects of the Shh signalling model in great detail. The auto proteolysis of Shh, its lipid modification, palmitoylation/cholesteroylation are fundamental to Shh dynamics over long distances. To a large extent, the movement of Shh from cell to cell is Tout-velu dependent transport. Previous research work has brought excellent scientific progress in unravelling the mysteries of Shh. We believe this brief informative review has enabled us to gain a comprehensive understanding on the key molecular events associated with Shh pathway and its mechanistic details. Further research, especially progress in genetic studies and development of novel biomarkers would reveal novel and intricate details of the biochemical and cell biological aspect of Shh signalling. More emphasis should be on understanding the mechanism of Shh activities eliciting altered cellular responses. The coming decade promises to reveal more to this dynamic signalling system.
The article complies with International Comittee of Medical Journal Editor’s uniform requirements for manuscript.
Competing interests – None,
Source of Funding – None
Received Date : 5 November, 2013
Revised Date : 11 January, 2014
Accepted Date : 25 February, 2014
References
1. Simpson F, Kerr MC, Wicking C. Trafficking, development and hedgehog. Mech Dev 2009; 126: 279–288.
2. Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature 2004; 432: 324–331.
3. Toftgard R. Hedgehog signalling in cancer. CMLS, Cell Mol Life Sci 2000; 57: 1720–1731.
4. Machold R, Fishell G. Hedgehog patterns midbrain ARChitecture. Trends Neuorosci 2002; 25: 10–11.
5. Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287: 795–801.
6. Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signalling molecules, is implicated in the regulation of CNS polarity. Cell 1993; 75: 1417–1430.
7. Roelink H, Augsburger A, Heemskerk J, et al. Floor plate and motor neuron induction by vHH-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell 1994; 76: 761–775.
8. Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent homeogenetic signals. Development 1993; 117: 205–218.
9. Chiang C, Litingung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 1996; 383: 407–413.
10. Nanni L, Ming JE, Bocian M, et al. The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: Shh mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet 1999; 8: 2479–88.
11. Ericson J, Briscoe J, Rashbass P, et al. Graded sonic hedgehog signalling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol 1997; 62: 451–457.
12. Jessel TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 2000; 1: 20–29.
13. Ericson J, Morton S, Kawakami A, et al. Two critical periods of Sonic Hedgehog signalling required for the specification of motor neuron identity. Cell 1996; 87: 661–673.
14. Marti E, Bumcrot DA, Takada R, et al. Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 1995; 375: 322–325.
15. Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol 2001; 11: 43–49.
16. Strigini M, Cohen SM. A hedgehog activity gradient contributes to AP axial patterning of the drosophila wing. Development 1997; 124: 4697–4705.
17. Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol 1995; 15: 2294–2303.
18. Porter JA, von Kessler DP, Ekker SC, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 1995; 374: 363–366.
19. Hammerschimdt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet 1997; 13: 14–21.
20. Porter JA, Ekker SC, Park WJ, et al. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996; 86: 21–34.
21. Pepinsky RB, Zeng C, Wen D, et al. Identification of a palmitic acid-modified form of Human Sonic hedgehog. J Biol Chem 1998; 273: 14037–14045.
22. Chamoun Z, Mann RK, Nellen D, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 2001; 293: 2080–2084.
23. Lewis PM, Dunn MP, McMahon JA, et al. Cholesterol modification of Sonic hedge-hog is required for long-range signalling activity and effective modulation of signalling by Ptc1. Cell 2001; 105: 599–612.
24. Zeng X, Goetz JA, Suber LM, et al. A freely diffusible form of sonic hedgehog mediates long-range signalling. Nature 2001; 411: 716–720.
25. Kohtz JD, Lee HY, Gaiano N, et al. N-terminal fatty-acylation of sonic hedgehog enhances the induction of ventral forebrain neurons. Development 2001; 128: 2351–2363.
26. Burke R, Mellen D, Bellotto M, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signalling cells. Cell 1999; 99: 803–815.
27. Chun H, Dong Y, Belenkaya TY, Xinhua L. Drosophila glypicans Dally and Dally-like shape the extracellular wingless morphogen gradient in the wing disc; Development 2005; 132: 667–679.
28. Mann RK, Beachy PA. Novel lipid modifications of secreted proteins signals. Annu. Rev. Biochem. 2004; 73: 891–923.
29. Hooper JE, Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell 1989; 59: 751–765.
30. Marigo V, Davey RA, Zuo Y, et al. Biochemical evidence that patched is the Hedgehog receptor. Nature 1996; 384: 119–20.
31. Charron F, Tessier-Lavigne M. Axonal pathfinding: Guidance activities of Sonic Hedgehog (Shh). In: Squire LR, ed. Encyclopedia of Neuroscience 2008: 1147–1153 [online]. Available at: http://www.elsevierdirect.com/brochures/ens/content.html
32. Taipale J, Cooper MK, Maiti T, et al. Patched acts catalytically to suppress the activity of Smoothened. Nature 2002; 418: 892–7.
33. Chen Y, Struhl G. In vivo evidence that Patched and Smoothened constitute distinct binding and transducing components of a Hedgehog receptor complex. Development 1998; 125: 4943–8.
34. Alcedo J, Ayzenzon M, Von Ohlen T, et al. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 1996; 86: 221–32.
35. Kandel ER, Schwartz JH, Jessel TM, eds. The induction and patterning of the nervous system. Principles of Neural Science. McGraw-Hill Professional, 2000.
36. Chen CH, von Kessler DP, Park W, et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell 1999; 98: 305–316.
37. Aza-Blanc P, Ramirez-Weber FA, Laget MP, et al. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell 1997; 89: 1043–53.
38. Cohen MM Jr. The hedgehog signalling network. Am J Med Genet A. 2003; 123: 5–28.
39. Philip WI, Andrew P. McMahon, Hedgehog signalling in animal development: paradigms and principles. Genes Dev 2001; 15: 3059–3087.
40. Kamal A, Goldstein LS. Principles of cargo attachment to cytoplasmic motor proteins. Curr Opin Cell Biol 2002; 14: 63–68.
41. Fukumoto T, Watanabe-Fukunaga R, Fujisawa K, et al. The fused protein kinase regulates hedgehog-stimulated transcriptional activation in drosophila schneider 2 cells. J Biol Chem 2001; 276: 38441–38448.
42. Nybakken K, Perrimon N. Hedgehog signal transduction: recent findings. Curr Opin Genet Dev 2002; 12: 503–11.
43. Stone DM, Hynes M, Armanini M, et al. The tumor-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 1996; 384: 129–134.
44. Robbins DJ, Nybakken KE, Kobayashi R, et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2 Cell 1997; 90: 225–234.