Annals of Neurosciences, Vol 17, No 1 (2010)
Annals of Neurosciences, Volume 17, Number 1, January 2010
Regeneration of tooth pulp and dentin : trends and advances
ABSTRACT
From the moment an egg is fertilized and a single cell zygote formed, it begins to divide and redivide, simultaneously differentiating into specialized cells that give rise to different body parts. A certain population of these cells which exhibit similar but limited potential to differentiate continue to exist in various tissues of the body throughout the lifetime of an organism. The science of tissue engineering exploits this valuable ability of stem cells/progenitor cells to regenerate or repair new tissues. In conservative dentistry, regenerative endodontic procedures aim to regenerate the pulp dentin complex utilizing dental stem cells, growth factors and scaffold matrix. Ability to isolate and harvest stem cells, improvements in the design of scaffold materials, advances in cell culture technology and commercial availability of growth factors has facilitated regeneration of pulp and dentin. Clinical techniques whereby these tissues can be regenerated include the surgical implantation of laboratory grown synthetic pulp and dentin tissues; implanting a mix of scaffold and growth factors and promoting endogenous recruitment of stem cells; or enhancing revascularization into root canals by encouraging stem cells to grow into natural fibrin clots. In this article, the components and techniques for pulp dentin regeneration will be discussed. Several preclinical studies have reported positive results but clinical cases reported in the literature are still very few. Additional research involving multiple disciplines that will translate these preclinical research outcomes into successful clinical applications need to be conducted. Every year a huge population of dental patients suffer from diseases like dental caries, which left untreated can progress to cause extensive loss of enamel and dentin with concomitant pulpal involvement. Currently, the treatment options available for a carious tooth are restoration of the defect with a filling material when the lesion size is small to moderate. In deep lesions extending close to a vital pulp, capping the pulp with a material which promotes pulpal healing and reparative dentin formation is the treatment of choice. In cases where the pulp is involved beyond repair, the dentist is left with no other option but to perform a root canal treatment which includes removing whole of the dental pulp and replacing it with an artificial filling material. Efforts have continuously focused to overcome limitations associated with existing restorative materials and evolve a material that mimics natural tooth structure as closely as possible. Clinical outcomes are also not always predictable especially in teeth with incompletely formed roots, resorption defects, fractures and extensive caries. Moreover, endodontic treatment leaves a tooth brittle and more prone to caries because the tooth is devoid of its blood supply, innervation and ability to produce secondary dentin. Developing a treatment therapy that will eliminate the use of any artificial material and enable replacement of lost tissues with natural pulp and dentin is an attractive option. Applying tissue engineering concepts can help turn this dream into reality. A combination of stem cells, scaffold and growth factors maintained in a controlled and regulated environment has shown immense potential to repair and generate tissues of the endodontium.
KEYWORDS: Tooth pulp, dentin, regeneration, stem cells, tissue construct, growth factors.
Corresponding Author: Sarang Sharma, MDS, E-mail : sarang74in@yahoo.com
doi : 10.5214/ans.0972.7531.2010.170109
Introduction
Structure of Pulpodentinal Complex
Dental pulp, a loose specialized connective tissue in the central portion of the tooth, is enclosed on its outer surface by dentin and comprises of cells, fibres, ground substance, blood vessels and nerves (Fig 1). Together they are known as the Pulpodentinal complex. The basic cells of the pulp are fibroblasts which lay down fibres of the pulp. Other cells present in the pulp include odontoblasts, undifferentiated mesenchymal cells, blood cells, endothelial cells, Schwann cells and cells of inflammation and immune reactions when present.1 Odontoblast cells are highly differentiated columnar cells arranged in a palisade along the outer border of the pulp. These cells send extensions into dentinal tubules called odontoblastic processes, which participate in dentin formation by secreting dentin matrix and minerals. The dentin matrix is primarily 90% collagen. Other non collagen matrix forming proteins include dentin sialo phosphoprotein (DSPP), dentin phosphoprotein (DPP) and Dentin matrix protein – 1(DMP 1). When calcium and phosphate minerals are subsequently deposited, the matrix is converted to mature calcified dentin. Histiocytes and undifferentiated mesenchymal cells are found localized around the vessel walls in the pulp and can differentiate into macrophages, fibroblasts, odontoblasts or osteoclasts under different cellular signals.Fibres provide support to the pulp and are predominantly type 1 collagen. Surrounding the fibres and the cells is the ground substance which permits transfer of nutrients, oxygen and wastes.
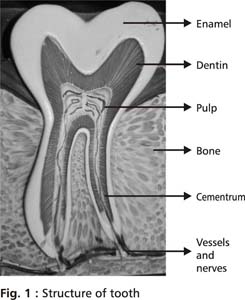
Dental pulp is richly innervated and vascular. Arterioles enter the dental pulp through the apical foramen along with the nerves, form a capillary plexus and drain into venules which exit from the apical foramen. The nerves entering the pulp through the apical foramen are both sensory and sympathetic.
The vascular system exists in intimate relationship with the nerves in the pulp and together they contribute to pulpal homeostasis. Arterioles in the dental pulp are heavily innervated by CGRP and SP containing sensory fibres. Activation of sensory nerves subsequent to a noxious stimuli increases the release of neuropeptides like substance P (SP), calcitonin gene related peptide (CGRP) and neurokinin A (NKA) which have shown to contribute significantly in regulation of pulpal blood flow and initiation of neurogenic inflammation by promoting angiogenesis, increasing vasodilation, enhancing vascular permeability and causing extravasation of immune and inflammatory cells into the site of injury.2 Denervation of sensory nerves has shown to cause a rapid necrosis of the exposed pulp because of impaired blood flow.2 Activation of sympathetic nerves releases neurotransmitters like nor epinephrine, adenosine triphosphate and neuropeptide Y (NPY) which cause reduction in pulp blood flow. Activation of parasympathetic nerves releases neurotransmitter like vasoactive intestinal peptide(VIP).
Developmental biology of tooth dentin and pulp
Tooth development, initiating at 6th week of intrauterine (I.U) life, is a process characterized by a series of complex reciprocal and sequential interactions taking place between oral epithelium and the underlying neuroectomesenchyme.4 Dental epithelium provides instructive signals for initiation of the tooth. Under the influence of cell signals, epithelial cells of the dental lamina at predetermined locations proliferate and project into the underlying neural crest derived ectomesenchyme.5,6 Initially this projection is in the shape of a bud, gets pronounced into a cap due to proliferation of cells. Further proliferation leads to the enamel organ assuming a bell shape. Simultaneous with the proliferation, there also occurs morphologic differentiation of cells in the enamel organ. The layer of cells on the inner side of the cap, inner enamel epithelium (IEE) differentiates into specialized cells called ameloblasts which lay down enamel. Layer of cells on the outer side of the cap is called outer enamel epithelium (OEE). The ectomesenchyme enclosed beneath the enamel organ condenses to form dental papilla whose outer most layer subjacent to the IEE differentiates into specialized cells called odontoblasts which lay down dentin (Fig 2). As dentin is laid down by odontoblasts, the existing dental papilla matures into dental pulp. Dental follicle is a mesenchymal tissue surrounding the developing tooth germ.
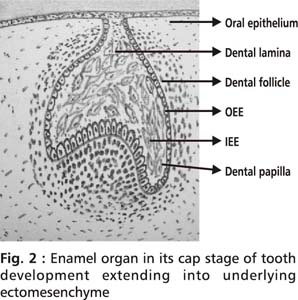
The inner and outer enamel epithelium grows apically to form a 2 cell layer called the Hertwig’s epithelial root sheath which commences root formation and also determines the shape of the roots.7 The Hertwig’s epithelial root sheath signals adjacent mesenchyme of the dental papilla to differentiate into odontoblasts which lay down root dentin and signals mesenchyme of the dental follicle to differentiate into cementoblasts (which form cementum), fibroblasts (which form periodontal ligament) and osteoblasts (which form alveolar bone).8
Key molecular events during this developmental process such as initiation, proliferation, morphogenesis, cytodifferentiation and spatial distribution of cells are principally controlled and mediated by the presence of morphogens/growth factors (GFs) like EGF, members of TGF family (BMP2-7, GDF),9 FGF family (such as FGF - 3, FGF - 4, FGF -8 and FGF -10),10 Wnt proteins (such as Wnt - 3, Wnt - 7b, Wnt -10a and Wnt 10b)11 and sonic hedgehog (Shh) proteins.5,6 Amongst several growth factors, BMP family of GFs seems to be the key factor in controlling tooth development by regulating various activities ranging from epithelial mesenchymal interactions, inducing mesenchyme to become odontogenic (BMP 4),12 controlling morphogenesis of epithelium to formation and maintenance of enamel knots (BMP 2, BMP 4 and BMP 7). Expression domain of BMP 4 has seen to expand with the loss of transcription factor Osr 2 leading to induction of supernumerary teeth.13 In Fgfr2b(-/-) mice, molar tooth development was seen to seize at the degenerated cap stage indicating its role in integrating tooth morphogenesis and dental axon patterning.14 Enamel deficiency, defects in cusps and root formation, abnormal dentin structure and excess number of teeth have been demonstrated in transcription factor, Epfn deficient mice.15
Molecular mechanisms behind reparative dentin formation
An insight into the natural molecular mechanisms behind pulpal wound healing in a tooth is essential as it enhances our understanding and serves as a backbone for successful regenerative endodontic procedures since both are based on similar biologic processes.
Pulpodentinal complex in its defense responds to an external stimuli or injury inflicted on the tooth by initiating inflammation or forming a protective barrier of tertiary dentin. Response of the pulp varies with the intensity and duration of tooth injury. In a mild injury, the already existing undamaged odontoblasts are stimulated to secrete reactive tertiary dentin. In injuries that are severe, odontoblasts may be either partially damaged or dead. Pulpal healing in severe injuries begins with the induction of apoptosis of damaged pulp cells. Progenitor cells / stem cells in the pulp particularly residing in the perivascular area and cell rich zone are stimulated to proliferate and migrate to the site of injury where they differentiate into secondary odontoblasts /odontoblastoid cells which lay down reparative tertiary dentin.16,17 Fibroblasts and pericytes may also be potential candidates for differentiating into odontoblast like cells.
The molecular control behind odontoblastic differentiation and reparative dentinogenesis is possibly attributed to differential gene expressions,18 protein activities19 and release of signaling molecules from injured pulpal cells. Dentin in addition is also a rich source of sequestered growth factors and bioactive molecules which are liberated when dentin matrix is demineralised/solubilized by bacterial acids during a carious attack or by certain dental materials like Ca(OH)2, MTA (Mineral Trioxide aggregate) and acid etchants used during some restorative treatment.20,21 Several growth factors released from dentin or secreted by pulpal cells shown to have potential regenerative effects16 include the TGF family, BMPs, non collagen proteins - DSPP and DMP 1,22 angiogenic growth factors like VEGF and human insulin like growth factor.23 TGF has shown to strongly express BSP ( bone sialoprotein), and DSP (dentin sialophosphoprotein) in exposed pulps of rat molars capped with MTA.24 Amelogenin gene splice products A+4 and A-4 induced reparative dentin formation, when these were implanted via dentin perforations into the pulps of maxillary first molars of rats.25 Several glucocorticoids have also been tested for their potential to regulate the formation of odontoblast like cells. Dexamethasone upregulates STRO-1, a marker of multipotent mesenchymal progenitor cells.26
Dentin repair is associated with an increased vascularity and initiation of innate immune responses in the area. This is attributed to the ability of pulp cells to secrete GFs that stimulate cell differentiation and neovascularization. Local increase in the vascular endothelial growth factor (VEGF), angiogenin, leptin,angiopoietin-2 (ANG-2), platelet derived growth factor (PDGF-BB) and heparin-binding epidermal growth factor (HB-EGF) promotes angiogenesis.27 Several other growth factors initiate and regulate angiogenesis either positively or negatively and either directly or indirectly.
Based on the same principles, provided there are growth factors, a scaffold template and nutrition, stem cells can be encouraged to differentiate into specialized cells and the desired tissue reconstructed using tissue engineering principles.
Tissue Engineering
Tissue engineering process involves developing an engineered construct in the laboratory, which is preceded by isolating stem cells from the body, expanding them in a culture and seeding these cultured cells on to nanofibrous scaffolds in culture medium supplemented with growth factors. The engineered tissue is then transplanted into the recipient site. Guided tissue regeneration differs from the former process in that a scaffold is implanted into the recipient site and endogenous stem cells from the systemic circulation or neighbouring sites are encouraged to inhabit the scaffold and form a new tissue.28,29 Three key elements critical to the success of tissue engineering include stem cells, morphogens / growth factors and a scaffold.30
Developments in the field of tissue engineering have made the generation of artificial substitutes in several areas of medicine a reality though focus is now more towards attaining structural, functional as well as biomechanical stability in these tissues destined for transplantation.31 Extensive research is being carried out in the field of dentistry as well. Iliac bone grafts have long been used in dentistry to regenerate furcation bone loss, dehisences and intraosseous periodontal defects.32 Guided tissue regeneration procedures, use of platelet rich plasma (PRP) and recombinant human bone morphogenetic protein (rh BMP) for bone augmentation are few procedures with good clinical outcomes. Though tissue engineering therapies have been used quite effectively in the area of periodontics, implantology and oral and maxillofacial surgery, their application in endodontics is still very limited.
Regenerative endodontics
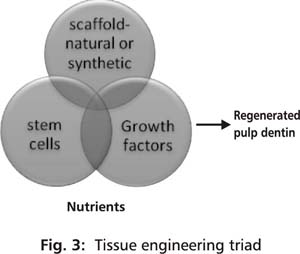
Regenerative endodontics involves employment of biologic procedures aimed at restoring or repairing damaged pulp dentin structures. For long, dentists have utilized calcium based biomaterials to stimulate calcific barrier formation in vital pulp therapy using pulp capping procedures. Surprisingly, this is one of the oldest regenerative dental procedures used clinically. Reparative dentin bridge which is formed under a pulp cap highly suggests the presence of precursor cells in the dental pulp of a tooth, that are capable of differentiating into secondary odontoblasts or atleast odontoblast like cells under appropriate signals.33 Recently, scientists have been successful in isolating stem cells from the dental pulps of permanent and deciduous teeth that have a potential to differentiate into specialized cells, are resourced easily and can be isolated and harvested conveniently. Discovery of these cells fitting into an appropriate stem cell source has raised hopes relating to regeneration of the tissues of the endodontium. Three essential components required to engineer pulp dentin tissue include an appropriate stem cell population, a physical scaffold and signaling molecules/growth factors supported by adequate nutrition (Fig 3). Nutrition in vitro is provided by the culture medium but when in vivo, in situ interactions with the host is a requirement. Cordeiro et al. were able to engineer tissue that resembled dental pulp using stem cells from human exfoliated deciduous teeth.34
Potential Stem cell sources for development of pulp and dentin
Stem cells are indispensable as they give rise to specialized cells required for normal growth and development of an organism. They are characterized by 1) ability to self renew and 2) give rise to daughter cells that eventually differentiate into multiple specialized cells. A small population of stem cells continues to exist in a quiescent state in the body throughout the life time of an individual and is crucial for maintaining health as it continually participates in the repair and replacement of damaged cells. Progenitor cells are another group of precursor cells that are capable of differentiation and proliferation but have a short life span compared to a stem cell and hence can reconstitute a tissue only for a short duration.
Two populations of stem cells involved in tooth formation are the epithelial stem cells and the mesenchymal stem cells; the latter required for pulp dentin regeneration. Mesenchymal stem cells have the potential to differentiate into tissues of mesodermal lineages - bone, cartilage, adipose tissue, skeletal muscle and connective tissue stroma. Mesenchymal stem cells can be derived from various sources in the body, but all of them do not exhibit similar phenotypes and expression profiles. Depending upon their tissues of origin, they may be already committed towards a specific differentiation pathway which could be because of their specific micro environments or niches. Mesenchymal stem cells located in a perivascular niche in the dental pulp, periodontal ligament, dental follicle and bone marrow may be potential sources for cell based therapies in regenerating the tooth.35,36
Autogenous post natal stem cells seem to be the most promising cells for regenerating pulp dentin tissues compared to embryonic and/or allogenic or xenogenic stem cells as they offer relatively easier accessible sources, reduce the possibility of immune rejection, pathogen transmission and have limited legal or ethical concerns. Exfoliating deciduous teeth, impacted third molars that need to be extracted or fractured teeth having exposed pulps that require endodontic treatment offer convenient ways of obtaining stem cells with limited ethical or legal concerns. The respective owner could opt to have the stem cells collected from these sources and stored for future regenerative dental treatments, if required. Creating stem cell bank facilitates prior proliferation and expansion of stem cells from a smaller sample size, alleviating the waiting period for this procedural step before a pulp organ construct can be made. The successful use of these stem cells is however a function of the culture conditions and micro environments used for maintaining and storing them. Following are a few mesenchymal stem cell sources that could be beneficial in regenerating pulp dentin complex.
Bone marrow stem cells (BMSCs)
Amongst various mesenchymal stem cells, BMSCs have served as the standard of comparison regarding multipotentiality and are the most extensively studied mesenchymal stem cells. Both BMSCs and DPSCs can differentiate into osteoblasts, chondrocytes, adipocytes and odontoblasts exhibiting the ability to generate osteoid or odontoid structures, although BMSCs display a lower odontogenic competence than DPSCs.37 BMSCs have demonstrated good ability to form tooth supporting periodontal structures like cementum, periodontal ligament and alveolar bone suggesting their potential use for treating periodontal diseases.38 However, because of their limited potential to generate odontoblasts, their use in pulp dentin regeneration may be limited and remains to be further explored.
Human Dental Pulp Stem Cells (hDPSCs)
Gronthos et al, were the first to successfully isolate a population of cells from human dental pulp which exhibited high proliferative capacity, multilineage differentiation potential, ability to self renew and form dentin pulp complex in Wfro.39,40 Dental pulp stem cells have gene expression profiles and differentiation capacity similar to BMSCs and under appropriate conditions can differentiate into osteoblasts, odontoblasts,41,42 chondrocytes and adipocytes though their ability to differentiate into adipocytes and chondrocytes is limited when compared to BMSCs. They also have an ability to differentiate into neural like cells under neurogenic induction medium.43
Dental Pulp stem cells/ progenitor cells are found to reside in the central cell rich zone of the pulp particularly in the perivascular and perineurosheath regions.35 Pericytes have also been suggested in some studies to be able to differentiate into odontoblasts.26 In an in vitro study by Huang et al, adult pulp cells after being seeded onto mechanically and chemically treated dentin surface demonstrated formation of cells having odontoblast like morphologies with processes extending into dentinal tubules.41 In another in vivo study, reparative dentin like structure was deposited onto the dentin surface when DPSCs seeded onto human dentin surface were transplanted into immunocompromised mice, suggesting the possibility of new dentin formation against existing dentin.44 The same study also provided evidence that osteogenesis and dentinogenesis mediated by BMSSCs and DPSCs respectively may be regulated by distinct mechanisms.
Research on hDPSCs has principally relied on isolation of cells from pulps of permanent third molar teeth.43,45 Huang et al, reported isolation and characterisation of hDPSCs from the pulp tissue of crown fractured teeth that did not require extraction as well as from supernumerary teeth thereby extending the potential available sources of hDPSCs.46 Compared to BMSCs, hDPSCs can be more easily sourced as they can be obtained from teeth that mandate pulp extirpation or from sound teeth that are deemed for extraction may be due to orthodontic or periodontal reasons.
Induced DPSCs compared to naive DPSCs may produce more regular dentinogenesis. This was observed when DPSCs isolated from rat dental pulp were co-cultured with tooth-germ-cell-conditioned medium.47
Stem cells from Human Exfoliated Deciduous teeth (SHED)
Exfoliating deciduous teeth contain living pulp remanants and are good sources of cells which are highly proliferative, clonogenic and have multi differentiation potential. These cells have been termed as stem cells from human exfoliated deciduous teeth (SHED) and were isolated and characterized by Miura et al.48 SHED offers attractive advantages over other post natal stem cells, as they are derived from a source which is non invasive, readily accessible, naturally being disposed and with very limited ethical or legal concerns. SHED is turning into a favorite choice for commercial stem cell banks where autologous stem cell sources may be stored for future use.
Compared to BMSCs and hDPSCs, SHED shows a higher proliferation rate and higher self renewal capabilities. They exhibit differentiation ability to convert into adipocytes, neural cells and odontoblasts but not into osteoblasts. They however exhibit an osteoinductive potential in which the host cells are stimulated to differentiate into bone forming cells.48 Miura et al, also demonstrated the inability of SHED to generate complete dentin-pulp like tissue as did hDPSCs, indicating that perhaps they are immature cells.48 Their use in regenerative endodontics needs to be further validated. Nor JE showed that SHED seeded onto synthetic scaffolds seated in pulp chamber of a thin tooth slice and implanted into immunocompromised mice generated odontoblast like cells against existing dentin.49 SHED seeded in biodegradable polylactic acid prepared within human tooth slice scaffolds and transplanted into immunodeficient mice were able to generate tissue with similarities to that of physiologic dental pulp while demonstrating capabilities to differentiate into odontolast like cells and endothelial like cells as well.34
Stem Cells from the Apical Papilla (SCAP)
Amongst various post natal stem cells, stem cells from the apical portion of the dental papilla of human immature permanent teeth happen to be a newly discovered population of stem cells by Sonoyama et al who termed them as stem cells of apical papilla (SCAP); and studied their physical and histological characteristics.50,51 It is known that dental papilla participates in tooth formation and later evolves into dental pulp. In immature teeth, when the roots are still developing, dental papilla assumes a position apical to the pulp tissue and the epithelial diaphragm.51 This apical papilla is loosely attached to the apex of the root from where it can be easily detached. In between the apical papilla and the overlying dental pulp is present a layer of highly populated cell rich zone. Apical papilla is less cellular and vascular compared to dental pulp but SCAP compared to DPSCs shows a proliferation rate higher by 2-3 folds. SCAP exhibits osteogenic, dentinogenic, adipogenic and neurogenic differentiation capabilities when exposed to the respective stimuli.
In an experiment conducted by Sonoyama et al, exvivo expanded SCAP in hydroxyapatite/tricalcium phosphate carrier when implanted into immunocompromised mice generated dentin like tissue on the surface of the carrier.50 Whether apical papilla is a cell source of primary odontoblasts that produce root dentin rather than replacement odontoblasts and if it is more suitable for pulp regeneration than DPSCs and SHED needs to be further investigated.
Neurogenic Potential of Dental Pulp Stem Cells
Non neuronal sources of stem cells that are capable of differentiating into neural cells may probably offer comparatively easier resources from which nerve cells could be regenerated. The fact that dental pulp stem cells are derived from neural crest mesenchyme raises hope that they may be good sources of stem cells to treat neural tissue injuries or degenerative diseases. Recent studies39,40,48,52 focusing on alternate stem cell sources for neural regeneration have demonstrated that DPSCS, SHED, SCAP and DFSCs possess differentiation properties of MSCs and NSCs. These stem cells have expressed a variety of neural cell markers like nestin, B lll-tubullin, GAD (glutamine and decarboxylase), Neu N ( neuronal nuclei), GFAP (glial fibrillary acidic protein), NFM (neurofilament M), NSE and CNP ase (2’3’cycle nucleotide- 3’ - phosphodiesterase) when induced with neurogenic medium. Miura et al, in their study were able to demonstrate the in vivo neural developmental potential of SHED.48 SHED when injected into the dentate gyrus of the hippocampus of immunocompromised mice survived for more than ten days and continued to express neural markers. In one study, dental pulp cells showed production of neurotrophic factors and were able to resume motor neurons after spinal cord injury.53
hDPSC cultures have shown to contain multipotent neural crest stem cells, which can differentiate into a number of neural crest derived cell lineages including melanocytes, and possess neurosphere forming abilities.54 Gangliosides may play a possible role in the neuronal differentiation process of hDPSCs. In a study when ganglioside biosynthesis was inhibited in hDPSCs by knockdown of UDP-glucose ceramide glucosyltransferase, differentiation into neural cells was prevented.55
Dental Follicle Stem Cells (DFSCs) also exhibit the ability to differentiate into neurons and can be isolated from follicle surrounding human third molars. Murine dental follicle precursor cells (mDFPCs) have demonstrated the ability to differentiate into neuron like cells similar to differentiated murine retinal progenitor cells (mRPCs), but have a limited differentiation potential into glia cells unlike mRPCs.56
Post natal stem cells isolated from dental anomalies such as odontomas have shown to be highly proliferative like DPSCs with stronger neural immunophenotypes than both DPSCs and mandible bone marrow stromal cells.57
Vascular regeneration
The ability to regenerate new microvessels anastomosis with the existing host vasculature is undoubtedly crucial for the survival of implanted or migrated cells and subsequently enhances the success of tissue engineering. Early development of vascularity improves the oxygen and nutrient inflow to the implanted stem cells.
Vasculogenesis is the formation of new blood vessels from endothelial cells that have differentiated from angioblasts (mesodermal precursor cells). The process results in establishing the primary vasculature in a tissue. Thereafter these vessels divide and form a complex network through sprouting of new tiny vessels from pre existing vessels, a process called angiogenesis. Various growth factors associated with inducing angiogenesis (acting directly or indirectly by regulating expression of other growth factors) include basic fibroblast growth factor (βFGF), transforming growth factor β (TGF-β), Platelet derived growth factor (PDGF), Vascular endothelial growth factor (VEGF) and TNF-alpha.58 Amongst all these growth factors, VEGF is the most important growth factor for physiological and pathological angiogenesis.
VEGF, a 45 kd heparin binding glycoprotein induces endothelial cell differentiation, proliferation, migration and survival.59 VEGF has 6 members in its family: VEGF A, VEGF B, VEGF C, VEGF D, VEGF E and platelet derived growth factor. VEGF A and B are closely associated with angiogenesis.60 VEGF is present in healthy dental pulp tissues61 and sequestered in the dentin matrix62 as well. Endothelial pulp cells respond to the morphogenetic and survival signals mediated by VEGF through receptors present on their surface promoting angiogenesis. Addition of angiogenic growth factors could promote establishment of vascularity in the construct.
In a study conducted by Cordeiro et al, organization of microvascular network in tissue construct was expedited, resulting in better organization of cells and greater cellularity when stem cells were coimplanted with human endothelial cells.34 Goncalves et al, demonstrated that sub-cutaneous placement of human pulpal tissue in immuno deficient mice promoted vascularization of the pulp tissue and subsequent angiogenesis in the area.63
Growth factors
Growth factors are polypeptides which along with their receptors regulate cell behavior and survival. Growth factors of concern in pulp dentin regeneration include the ones that will aid differentiation of stem cells into odontoblasts, promote matrix secretion and mineralization, angiogenesis and neurogenesis. Those that have been studied extensively and have shown to have significant effect in regeneration include the TGF-β family, BMP family, FGF, non collagenous proteins-DMP1, DSP, DPP and VEGF.
Transforming growth factor beta family exists in three isoforms: TGF β1, TGF β2 and TGF β3 and is produced by pulp cells and odontoblasts as well as found incorporated in the dentin matrix.62 They regulate various activities ranging from proliferation, migration and differentiation of stem cells/progenitor cells into odontoblasts, stimulation of dentin matrix secretion to promoting angiogenesis.64 TGF β1 has shown to enhance reparative dentin formation in vivo65 and TGF β2 has shown to stimulate the synthesis and deposition of matrix.66 TGF βs1-3 stimulated differentiation of adult pulp stem cells into odontoblast cells in cultured tooth slices.64 However, in a study conducted by Tai et al, TGF β2 inhibited the growth and differentiation of pulp stem cells.67 Variable responses of TGF β2 on dental pulp cells can be attributed to the activation of multiple signaling pathways that may signal different responses. More research needs to be conducted as information about TGF βs signaling on the growth and differentiation of pulp stem cells is still scarce and diverse. The effect of concentration, presence/absence of other GFs and differentiation stages of cultured pulp cells on the expression of TGF βs need further clarification.
Bone Morphogenetic Proteins (BMP 2-7) belong to the TGF β superfamily of proteins, are produced by odontoblasts and are found incorporated in the dentin matrix as well. These are produced using recombinant DNA technology and interact with specific receptors on the cell surface referred to as the bone morphogenetic protein receptors. Rh BMP-2 stimulates differentiation of adult pulp stem cells into an odontoblastoid morphology in culture.68 Recombinant BMP-2, -4 and -7 promotes pulp cells to differentiate into odontoblasts and induce reparative dentin.69,70 The effects of recombitant bFGF, insulin like growth factor(IGF)-II and TGF β1 on dental pulp cells of mechanically exposed pulps in dog teeth were compared by Tziafas et al, who demonstrated TGF β1, to have better odontoblast inducing abilities than FGF and IGF.71
Fibroblast growth factor-2 (FGF-2) displays potent angiogenic activity and mitogenic ability on mesenchymal cells. It plays an important role in physiologic enamel and dentin formation and is normally stored in the extracellular matrix. Reparative dentine was formed when FGF-2 was incorporated on amputated rat molar pulps; however the dose of FGF released at the site showed significant effect on the nature of calcified tissue formed.72,73
Dentin matrix protein 1(DMP1), dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) are non collagenous extracellular matrix proteins of the dentin and bone, out of which DMP1 serves as a potent growth factor. It is implicated in the differentiation of odontoblastic progenitor cells and regulation of mineralization.74 Prescott et al, successfully extracted and purified DMP1 for use in dental tissue regeneration. They showed that the triad of DPSCs, collagen scaffold and DMP1 was able to induce an organized matrix formation similar to that of pulp tissue.75 Almushyat et al, have demonstrated formation of dentin like hard tissue when dental pulps in rat models were capped with collagen scaffolds seeded with DMP1,22 They were able to induce differentiation of DPSCs into odontoblasts. In presence of DMP1, mineral deposition has seen to increase by 10 fold.74 Enamel matrix proteins-90% amelogenins and 10% non amelogenin proteins have shown to induce mesenchymal cell differentiation and/or maturation of odontoblast precursor cells residing in the dental pulp into odontoblast like cells.76 The role of EMD in periodontal ligament regeneration and cementogenesis in patients with periodontitis is well documented.77
Scaffold
Scaffold is a three dimensional biodegradable porous polymer framework that serves as a potential biologic carrier to facilitate delivery of stem cells and/or growth factors at a local receptorsite.lt provides a matrix for cell seeding, cell adhesion and growth. Nutrients may be embedded into the scaffold to promote cell survival. To serve as a physical matrix for pulp dentin reconstruction, the scaffold should meet certain requirements like ease of handling, conductive for odontoblast like cells, adequate porosity, biodegradability, biocompatibility, good physical and mechanical strengths and ability to support vascularity. Adequate pore size and pore volume is desired in a scaffold to permit penetration and diffusion of cells as well as growth factors.78 A scaffold serves to function as a temporary structural framework until replaced by new matrix formed by inhabitant cells capable of supporting the load. This means that the rate at which a scaffold is degraded by the surrounding tissues should be in approximation with the rate at which the new tissue is formed. Also it should degrade without leaving behind any noxious byproducts.
A tooth is routinely subjected to mechanical loads through out the day, hence it is crucial that a matrix particularly proposed for regenerating dentin has adequate mechanical properties to support the applied loads. Additionally, the regenerated matrix should not show any volumetric change lest it induces residual stresses in the tooth subjecting it to fracture.
Synthetic or natural polymers can be used as scaffold materials in pulp dentin regeneration. Use of synthetic polymers has long been popular in medicine as resorbable sutures. Several synthetic polymers that have been successfully used as a scaffold matrix because of their biocompatibility and biodegradability include polyester materials like lactic acid (PLA), polyglycolic acid (PGA), poly lacticco-glycolic acid (PLGA), and polycaprolactone (PCL).79,80 These polymers degrade to form lactic acid or glycolic acid, a natural chemical which is easily removed from the body. Their use is approved by the FDA and are easy to fabricate. Other synthetic polymers that have been advocated as scaffold materials include hydrogels, calcium phosphates, glasses and composites. Hydrogels like polyethylene glycol polymers and hydroxypropylmethyl cellulose polymers in contrast to former synthetic polymers are non rigid and can be injected.81 PGA has shown to be a more conducive scaffold for development of a tissue with cellularity similar to dental pulp than a hydrogel or aIginate.82
Calcium phosphate (Ca/P) scaffolds include Beta-tricalcium phosphates(β-TCP) or hydroxyapatite (HA). Ca/P can be suitable for dentin regeneration as it is similar to the natural mineralized tissue, is biocompatible and osteoinductive. Hench et al formulated and suggested the use of bio active glass in tissue engineering. It could serve as a potential scaffold material due to enhanced odontoblastic activity on its surface.83 A composite scaffold of polymer and Ca/P could harness the advantages of both materials overcoming the shortcomings of individual material.84 DPSCs seeded onto 3-D collagen, ceramic and titanium mesh scaffolds when implanted into nude mice formed a tissue that resembled connective tissue.85
Natural scaffold materials tried are proteins like collagen or fibrin, polysaccharides like chitosan or glycosaminoglycans and alginates.75 They offer advantages of bioactivity and biocompatibility though may initiate some immune reactions. These polymers also have good cellular compatibility i.e. are able to support cell survival and function. Because collagen has a good tensile strength, it can be twisted or weaved into desired forms. Collagen is a predominant component of dentin and pulp tissues. Collagen scaffolds have hence been popularly used in regenerative studies because of their structural similarity to natural tissues. When polylactic acid polymer, collagen and calcium phosphate materials were compared as scaffolds, polymer and collagen showed significantly more DPSC survival than calcium phosphate scaffold.86
Another material of interest is Platelet rich plasma (PRP) and Platelet rich fibrin (PRF) which could serve as potentially viable scaffold materials as they are rich in preexisting growth factors like PDGF, TGF β, are biodegradable and easy to prepare in a dental setting.87 Based on the finding that dentin chips can stimulate dentin formation in an exposed pulp, these may be considered as a scaffold matrix.88 They provide a suitable matrix for pulp stem cell adherence and are a reservoir of sequestered growth factors which promote differentiation of DPSCs into odontoblasts.41
A single matrix may not be an ideal scaffold material. Combination of two or more materials may be the best suited matrix material.
Possible regenerative endodontic procedures
Capitalizing on the aforementioned principles of tissue engineering, technologies for regenerating endodontium can be based on two approaches.30,89,90
(1) Creating a denovo engineered tissue construct in the laboratory and transplanting it into the recipient tooth.
(2) Inducing host stem cells from the adjacent site to mobilize and inhabit the implanted/natural host matrix.
It is emphasized here that most of these technologies are hypothetical and do not currently have FDA approval for use in patients.
Regenerating Pulp
Creating and transplanting tissue construct into the pulp cavity
Transplanting pulp stem cells without a matrix into the root canal may fail to generate a new tissue because of the inability of the cells to survive and populate an empty canal space. Another unfavorable outcome could be the uncontrolled migration of stem cells to different areas of the body leading to aberrant patterns of mineralization; hence the need to localize stem cells and aid their attachment which is possible using a scaffold matrix. With the advances in tissues engineering techniques, it is now possible to seed stem cells onto a three dimensional conductive scaffold matrix rich in bioactive signaling molecules and grown in an appropriate culture medium. This artificial pulp construct generated in the laboratory can then be transplanted into the pulp cavity.
Gebhardt et al, showed that pulp constructs could be created using polymer and collagen scaffolds seeded with DPSCs.86 Both these scaffolds showed better cell survival compared to calcium phosphate scaffolds. Gotlieb et al, also concluded that pulp tissue constructs made with polylactic acid polymer and collagen seeded with SHED could be implanted into cleaned and shaped root canals.91 However in certain studies, collagen scaffolds impregnated with pulp cells have shown to undergo significant contraction both in vivo and in vitro which could affect pulp tissue regeneration.92,93 PLG matrices seeded with DPSCs have shown good cellular adhesion with no observable contraction suggesting their use as a suitable carrier.92 Zhang et al, observed successful dental pulp stem cell growth on spongeous collagen, porous ceramic and fibrous titanium mesh scaffolds that were implanted into nude mice.85 Further research needs to be conducted to identify an optimum matrix deemed suitable for carrying pulp stem cells and maintaining their survival.
Supplementation of culture medium with local growth factors promotes stem cell proliferation and differentiation. Dosage of the growth factor incorporated and released from a matrix may however influence the structure of calcified tissue formed.73 Controlled release of GF is possible by incorporating it into a gelatin hydrogel which gradually releases GF during in vivo biodegradation.73,94 The differential inducibilityof stemcellsfrom different species may vary with different methods used in culture which could affect the clinical outcomes of tissue engineering therapies.95
Merely combining all three elements generates a tissue with haphazardly distributed cells and a questionable functional capacity. To overcome these deficiencies and in the hope of creating a more structured and functional tissue, an engineered three dimensional model is advocated in which layers of growth factors and cells can be organized in a sequence such that the future desired pattern is attained that is odontoblastoid cells in the periphery and fibroblasts, vessels and nerves in the core of the pulp. Devices however need to be developed that will enable 3D construction of the scaffolds and allow precise placement of the cells.
The ultimate aim is to generate a core of pulp tissue surrounded by newly formed odontoblasts that grow on root canal dentin wall as well as produce new dentin onto existing old dentin. The clinical success of generating such a functional pulp tissue will depend upon the attachment of pulp stem cells to the scaffold matrix as well as to the surrounding pulp cavity surfaces, and integration of the implanted construct with the host vasculature. Pulp tissue constructs fabricated from SHED and polylactic acid scaffolds with/without added GFs-BMP-2 and TGF β1 have shown a more complete adherence to the coronal dentin and a less complete adherence to the middle and apical aspects of the root canal,91 which is likely due to the complex anatomy of the root canal towards the apex and the physical constraints of the placement method in narrow canals at the apex. In a recent study, pulp like tissue was shown to regenerate in emptied root canal space, with odontoblast like cells producing dentin like tissue on existing dentinal walls; when SCAP and hDPSCs seeded onto poly lactide/glycolide scaffolds were inserted into tooth fragments and transplanted into immunocompromised mice.98
Delivering engineered tissue into pulp cavity
Implantation of engineered 3D tissue construct into a pulp cavity seems to be a very attractive approach but demands construction of precise 3D models for each individual pulp cavity. Highly complex and variable internal anatomy amongst 32 teeth with variations from tooth to tooth and individual to individual makes the task quite ardent. Moreover, an effective viable delivery system that will allow introduction of these constructs into extremely minute canal spaces will have to be developed.
On the occasion of 85th general session of the international association for dental research, scientists reported on high internal phase emulsion (HIPE) polymerization which affords tremendous control of scaffold morphology. HIPEs can be readily moulded into irregular shapes and cured in situ to a rigid foam with interconnected pores. These can be of interest in regenerative endodontics because of the rigidity of the resulting foams and the relative ease of fabrication.
Injecting a soft scaffold matrix impregnated with pulp stem cells and GFs into areas with accessibility constraints like the root canal system can overcome difficulties associated with implanting a rigid matrix. Scaffold material that can be injected includes synthetic hydrogels like poly ethylene glycol polymers.81 Though delivery system is relatively easier with these polymers, problems of low cell survival and limited control over tissue formation still exist. Modifying hydrogel polymers with peptides like arginine, glycine or aspartic acid have helped in improving cell adhesion and matrix synthesis rendering them suitable for use.96 Making the hydrogels photo polymerizable so that they form a rigid framework after implantation into the receptor site is a more practical solution.97 The ability to use this system in vivo and functional results still need to be further researched.
Whether the engineered tissue is implanted or injected, there always exists a possibility of bacterial ingrowth into the root canal system. Thorough disinfection is essential for cell survival and integration of dental pulp construct with the host tissues. This mandates that the receptor site should be thoroughly disinfected. Additionally, antibiotics impregnated into the scaffold matrix possibly by using nano engineering techniques can prevent bacterial growth.99
Another persistent problem which needs to be addressed is the lack of initial blood supply making sufficient oxygen and nutrients unavailable for the implanted cells. In the laboratory, extensive culturing with closely monitored oxygen, pH, humidity, temperature, nutrients and osmotic pressure promotes cell survival, growth and functionality. When transplanted in vivo, blood vessel ingrowth is important to provide nutrition which in a tooth can occur only through the apical foramen present at the root apex as a tooth lacks collateral circulation. With increasing distance from the apex, cell survival becomes difficult because of the decreasing nutrient supply. Introducing engineered tissue in multiple increments may help overcome this limitation but at the same time would increase the possibility of bacterial contamination due to re entry into the tooth. Addition of angiogenic growth factors may render it possible to transplant tissue in a single increment. In anterior teeth, where the root canals are large or in teeth with immature roots, angiogenesis may be easily achieved. But how far angiogenesis is possible in posterior teeth where the canals are extremely narrow still needs to be experimented. Prior enlargement to a suitable size so as to receive tissue may be deemed necessary in narrow canals.
Gene enhanced tissue engineering
Gene based therapies for tissue regeneration involve introduction of genes into target cells with the aim of altering their phenotype or protein expression profile. Transfection is the use of chemical (calcium phosphate, lipids, polymers and proteins) or physical (electropolation, microinjection, naked DNA) methods for introducing DNA into recipient cells where as transduction is the use of viral vectors for introducing DNA.100
Virus for this purpose is rendered noninfectious by deleting key genes from the viral genome that prevent its replication. This therapy is beneficial in obtaining sustained production of growth factors at physiologic levels especially when growth factors have short half lives. Another approach could be to introduce mineralizing genes in the pulp tissue to induce calcific tissue formation.
Successful bone induction has been reported after application of the BMP family members BMP-2, BMP-4, BMP-7 and BMP-9 by gene therapy using viral vectors.101 Transplantation of cultured dermal fibroblasts expressing virally mediated BMP has induced reparative dentin formation in exposed pulps with reversible pulpitis.102 Mineralized tissue has shown to effectively form after BMP 2 transfected DPSCs seeded onto ceramic scaffolds were implanted into nude mice.103
The cost effectiveness and long term safety of this treatment therapy however needs to be evaluated. Prolonged expression by integration of the gene/viral vector into the target cell genome can result in tumor formation. More research is required to devise ways by which the vector is targeted at the desired cells only, thereby minimizing health risks. Regulatory approval for safety concerns and choice of delivery system is essential before gene therapy can be widely used. Most of the gene therapies are not widely researched and the disadvantages and limitations are mainly theoretical. Whether the entire process of gene enhanced tissue engineering can be performed in a clinical setting without the need of any specialized equipment or extensive training still needs extensive exploration.
Pulp regeneration by inducing endogenous stem cells to inhabit a matrix
Generation of pulp tissue within the root canal space by inducing stem cells from the adjacent site to populate the area can be possibly achieved if the following coexist (1) thorough disinfected canal (2) presence of a matrix within the canal on which new tissue can grow (3) appropriate factors or stimuli present locally in the desired concentration and for desired time period and (4) bacteria impermeable seal of the access opening. There are several case reports which have documented regeneration of vital tissue in immature teeth with incompletely formed roots after the canals were thoroughly disinfected and bleeding was established in the canals by over instrumentation. This process has been termed as pulpal revascularization by mostauthors.104,105,106
Irrigation with sodium hypochlorite (0.5 – 5.2%), hydrogen peroxide (3%) or chlorhexidine gluconate with/without the additional use of antibiotics106,107 is used to achieve a high level of disinfection. However, there is a need to use biocompatible irrigants that will promote stem cell attachment onto root canal dentin without being cytotoxic. The comparative efficacy of 10 different endodontic irrigant solutions on dental pulp stem cell attachment to root canal surfaces has been studied and results have shown that attachment of DPSCs correlates with the cytotoxicity of the irrigant solutions.108 A combination of ciprofloxacin, metronidazole and minocyclin has shown promising results in effectively eliminating bacteria from carious lesions, infected pulp and infected root dentin.109 Tetracycline may have an additional possible effect of enhancing growth of pulp cells onto dentin by exposing embedded collagen or growth factors in dentin,110 though contribution of this effect by minocycline in revascularization still needs to be studied.
Significance of a matrix loaded with growth factors has already been emphasized. Inducing a blood clot to form in the canal yields a natural fibrin matrix rich in constituent growth and differentiation factors. Thiobodeau et al, compared the efficiency of artificial collagen solution, blood clot, and a combination of collagen and blood as matrix materials for enabling revascularization in root canals of immature dog teeth. The inclusion of a blood clot was highlighted to obtain desirable results.104 PRF / PRP is a platelet concentrate and can be added to enrich the natural blood clot. Several GFs derived from platelets like PDGF, insulin like GF 2, platelet factor 4 and TGF β not only act directly on the tissue but also interact with other GFs to activate gene expression and protein production.87,111 A bacteria impermeable seal of the access opening provided by an adequate thickness of restorative material is mandatory to prevent any bacterial infiltration and destruction of the neo tissue.
Appropriate clinical outcome of this treatment can be determined by radiographic demonstration of continued root development, lateral thickening of canal walls and healing of periapical radiolucency, if any. Clinically, there is absence of any signs/symptoms and occasional positive response to pulp testing may be elicited but the latter may not be very reliable. More reliable methods to determine vitality are to use laser Doppler flowmetry or magnetic resonance imaging but these have not become very popular because of their expense involved. Histologic analysis of the regenerated tissue may not be possible in human patients because reentering the tooth would need informed consent, strict inclusion criteria and may not be ethically acceptable. A direct histologic evaluation may be possible only in animal models or in humans if at some later stage the treated tooth requires extraction perhaps due to periodontal reasons or pulp extirpation needs to be performed as part of the root canal treatment. The incidence of such cases reporting in future to the same treating doctor may be very low and a general conclusion cannot be based on isolated case analysis.
The cellular source of regenerated tissue and precise mechanisms behind biologic root development still needs to be identified. Whether the regenerated tissue is periodontal ligament tissue which grows into the canal from the apical foramen and lays down cementum onto the inner walls of the root canal or is pulp tissue which has grown from surviving pulp stem cells in the lateral canals or from SCAP is still not clear. It is likely that SCAP residing in the apical papilla is able to survive the infection because of its proximity to the highly vascularised periodontal tissue. These pulp stem cells may rebuild a lost pulp tissue but whether it contains functional odontoblasts that get attached to the canal wall dentin and lay new dentin is still questionable. Newly deposited hard tissue may have a histologic picture of cementum, bone, dentin or osteodentin. In a recent study, Wang et al who conducted histologic evaluation of the type of tissues that had grown into the canal spaces of dog teeth however concluded that the thickening of the canal wall and the apical bridge so formed was due to the apposition of intracanal cementum and connective tissue found in the canal space was similar to periodontal ligament.112 More long term histologic studies need to determine the type of tissue generated, its effect on the clinical outcome and the fate of tooth receiving such kind of treatment.
Revascularization in almost all cases has been attempted in teeth with immature roots where the apex is wide enough to permit abundant vascular ingrowth. Teeth with closed apices may warrant the need for deliberately perforating the apex to a suitable size of 1-2mm so as to promote ingrowth of blood vessels. Studies need to be conducted in animal models and the advantages/disadvantages of revascularization treatment compared to conventional root canal treatment before the former can be routinely attempted in human mature teeth. Clinical outcomes when attempting revascularization may vary with age and the type of tooth. These variables also need to be addressed.
Regenerating Dentin
Vital pulp therapy which has long been practiced involves regenerating dentin by placing osteoinductive material in a deep tooth cavity. The limitation however associated with this therapy is that the defect is not filled with dentin tissue but the dentin is formed towards the pulp chamber constricting its size. So far, calcium based materials have been popularly employed. Use of a pulp capping material with growth factors incorporated into the capping material could possibly accelerate and improve the quality and quantity of reparative dentin formed. The best alternative however would be a treatment therapy which enables replacement of lost dentin with regenerated dentin tissue that integrates with pre existing dentin. This will not only eliminate the bacterial prone interface but will also restore the strength of the tooth as it functions as a single unit. Implantation of a scaffold enriched with growth factors on an exposed pulp site may promote growth of stem cells into the scaffold and convert the scaffold into dentin (Fig 4). The large cavity thereby gets filled with dentin reducing the size of the defect.
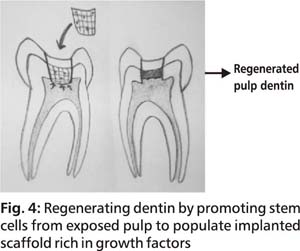
A thorough disinfection is however advocated before any implantation is carried out. All caries affected dentin should be excavated as presence of any micro organisms will inhibit the migration and differentiation of pulp stem cells. Restorative material used above the tissue construct should also provide a thorough sealing at the tooth margins. Any leakage at the tooth restoration interface would affect dentin regeneration. Statin in adequate doses could be included in the scaffold to accelerate differentiation of DPSCs.113 Statin, 3-hydrohy 3methylglutaryl coenzyme A reductase inhibitor, is known for its anti inflammatory effect as well as increasing angiogenesis and neurogenesis. Reactive oxygen species and hydroxyl radicals in low quantities have also shown to be beneficial by being bactericidal and enhancing proliferative, differentiation and calcific activity of DPCs. Irrigation with low concentration H2O2 prior to placement of the scaffold cap could aid in dentinogenesis.114
Ishimatsu et al placed a scaffold of collagen sponge impregnated with FGF-2 on the dentin defect above amputated pulps of rat models.73 Pulp stem cells and vessels were seen to invade the collagen sponge during the early phase. In the late phase, calcified tissue was formed when moderate doses of FGF-2 were incorporated into gelatin hydrogels. High doses of FGF-2 formed isolated calcified particles where as low doses of FGF-2 formed no dentin barrier suggesting that the dosage of released FGF-2 had an influence on the structure of calcified tissue regenerated in dentin defects. The effect of incorporating other GFs and their doses on the amount and nature of calcified tissue formed needs to be further studied. In a recent study, when tissue constructs made of HA/TCP scaffold seeded with dental pulp derived cells were transplanted onto exposed pulps of porcine molars, a large quantity of regular calcified dentin was seen to form. When only scaffold was placed on the exposed pulp, hard tissue was formed but without odontoblasts and tubule like structures suggesting that scaffold should be loaded with cultured pulp cells before placement.115
Any change in the volume of the scaffold can induce physical stresses within the tooth. Scaffolds used should therefore be ones that will not show any dimensional change. The size of pulp exposure site may also affect the invasion ability of the vessels and cells. A small sized exposure may not permit adequate invasion. Moreover, dentin regenerative ability of pulp varies with age affecting the prognostics of rendered treatment. In young DPCs, self defense is achieved more by calcification compared to adult DPCs where it responds more by vessel regeneration.116 More research needs to be carried out to determine the effect of age and size of exposure on the regeneration of dentin.
Conclusion
The success of root canal treatment in endodontics is close to 90% with a good long term prognosis and ease of performance in a clinical setting. Treatment procedures that will enable regeneration of endodontium will have to be cost effective, clinically practical, non lengthy and patient conducive so as to enable them to be performed on a routine basis. The success rate of regenerative endodontics with its long term prognosis needs to be evaluated and improved before it can replace the conventional root canal treatment and restorations. In spite of the directed efforts in dealing with various complexities associated with regenerating tissues, the field is still largely in its infancy.
The success of regenerative endodontics will depend on how closely multidisciplinary teams of clinicians, engineers, scientists and technicians can work, each contributing his own area of expertise to expand research. There is an extensive need to translate preclinical research into clinical realities. Government should encourage and fund the growth of small – moderate sized scientific enterprises with highly qualified personnel that could exploit and develop the ongoing research into clinically viable technologies.
Forums should be formed by which various agencies involved in tissue science and engineering stay informed of each other’s activities and are hence able to coordinate their efforts in a timely and efficient manner. These agencies can help promote the entire field of tissue engineering without wasteful duplication of efforts by creating common websites, organizing scientific meetings and workshops, monitoring technology by undertaking cooperative assessments and advertising for new opportunities.
Even though it is a long way to go, once the potential of regenerative endodontics is unleashed, it would be of immense clinical advantage and benefit millions of patients as an enormous part of dentistry is based on treating patients with dental caries.
Competing interests: None
Received Date : 16 December 2009
Revised Date : 13 January 2010
Accepted Date : 28 January 2010
References
1. Nanci A. Dentin-Pulp Complex. In : Ten cate’s Oral Histology, Development, Structure and Function, 7th ed. Mosby: Elsevier Press 2008; 191-238.
2. Caviedes-Bucheli J, Munoz H, Azuero- Holguin M et al. Neuropeptides in dental pulp: The silent protagonists. J Endod 2008; 34(7): 773-788.
3. Cain JH, Baggott C, Tilghman Jl, et al. Recent developments in the study of spinal cord injury and neuropathic pain. Annals of Neurosciences 2007; 14(4): 96-107.
4. Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development 1988; 103: 155-169.
5. Cobourne M, Hardcastle Z, Sharpe PT. Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J Dent Res 2001; 80(11): 1974-1979.
6. Sarkar L, Cobourne M, Naylor S et al. Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci USA 2000; 97: 4520-4524.
7. Oshima H. Overview: Developmental biology of hertwigs epithelial root sheath (HERS) and tooh root formation. J of Oral Biosciences 2008; 50(3): 147-153.
8. Nanci A. Development of the tooth and supporting structures. In : Ten cate’s Oral Histology Development, Structure and Function, 7th ed. Mosby: Elsevier Press 2008; 79-107.
9. Nadiri A, Kuchler-Bopp S, Haikel Y et al. Immunolocalization of BMP-2/-4, FGF-4 and WNT10b in the developing mouse first lower molar. J Histochem Cytochem 2004; 52(1): 103-112.
10. Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 1998; 125: 4325-4333.
11. Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol 1998; 202(2): 215-227.
12. Vainio S, Karavanova I, Jowett A et al. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 1993; 75(1): 45-58.
13. Mikkola M. Controlling the number of tooth rows. Science Signal 2009; 2(85): 53.
14. Kettunen P, Luukko K. Fgfr2b signaling integrates tooth morphogenesis and dental axon patterning. Dev Biol 2008; 319(2): 508.
15. Nakamura T, De Vega S, Fukumoto S et al. Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J of Biological Chemistry 2008; 283: 4825-33.
16. Cogliati T and Swaroop A. Stem cells and neuronal repair. Annals of Neurosciences 2009; 16(4): 143-145.
17. Smith A, Patel M, Graham L, et al. Dentin regeneration: key roles for stem cells and molecular signaling. Oral Biosciences and Medicine 2005; 2(2): 127-132.
18. Srivastava NK, Yadav R and Pradhan S. Metabolic myopathies: clinical, biochemical, genetic and histopathological basis of diagnosis. Annals of Neurosciences 2005;12 (4):87-98.
19. Keshava R, Jothi M, Gope ML. Functional modulation of the p53 gene and its protein in human brain tumors. Annals of Neurosciences 2008; 15(3): 75-86
20. Graham L, Cooper P, Cassidy N et al. The effect of calcium hydroxide on solubilization of bioactive dentin matrix components. Biomaterials 2006; 27(14): 2865-73.
21. Smith A, Lumley P, Tomson P. Dental regeneration and materials – a parternership. Clinical Oral Investigations 2008; 12(2): 103-108.
22. Almushayt A, Narayanan K, Zaki A et al. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Therapy 2006; 13(7): 611-620.
23. Sharma NK, Prabhakar S, Anand A. Age related macular degeneration advances and trends. Annals of Neurosciences 2009; 16(2): 62-71.
24. Hwang Y Hwang I, Oh W et al. Influence of TGF 9461 on the expression of BSP, DSP, TGF 9461 receptor I and Smad proteins during reparative dentinogenesis. J of Mol Histol 2008; 39(2): 153-160.
25. Six N,Tompkins K, Septier D et al. Recruitment and characterization of the cells involved in reparative dentin formation in the exposed rat molar pulp after implantation of amelogenin gene splice products A+4 and A-4. Oral Biosciences and Medicine 2004; 1(1): 35-44.
26. Alliot-Licht B, Bluteau G, Magne D et al. Dexamethasone stimulates differentiation of odontoblast- like cells in human dental pulp cultures. Cell and Tissue Res 2005; 321(3): 391-400.
27. Karim I, linden G, Irwin C et al. Neuropeptides regulate expression of angiogenic growth factors in human dental pulp fibroblasts. J Endod 2009; 35(6): 829-833.
28. Bianco P, Robey P. Stem cells in tissue engineering. Nature 2001; 414: 118-121.
29. Seppala M, Zoupa M, Cobourne M. Tooth development : 2. Regenerating teeth in the laboratory. Dental Update 2007; 34; 20-29.
30. Murray P, Garcia-Godoy F, Hargreaves K. Regenerative endodontics: A review of current status and a call for action. J Endod 2007; 33(4): 377-390.
31. Praveen S, Krishnakumar K, Sahoo S. New era in health care : tissue engineering. J of Stem Cells & Reg Med 2008; 1 (1).
32. Schallhorn R, Hiatt W, Boyce W. Iliac transplants in periodontal therapy. J Periodontol 1970; 41: 566-80.
33. MitsiadisT, Rahiotis C. Parallels between tooth development and repair: conserved molecular mechanisms following carious and dental injury. J Dent Res 2004; 83(12): 896-902.
34. Cordeiro M, Dong Z, Kaneko T et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 2008; 34(8): 962-969.
35. Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J bone Miner Res 2003; 18(4): 696-704.
36. Seo B, Miura M, Gronthos et al. Investigation of multipotent stem cells from human periodontal ligament. Lancet 2004; 364(9429): 149-155.
37. Yu J, Wang Y, Deng Z et al. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell 2007; 99: 465-474.
38. Kawaguchi H, Hirachi A, Hasegawa N et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 2004; 75(9): 1281-1287.
39. Gronthos S, Mankani M, Brahim J et al. Postnatal human dental pulp stem cells(DPSCS) in vitro and in vivo. Proc Natl Acad Sci USA 2000; 97: 13625-13630.
40. Gronthos S, Brahim J, Li W et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002; 81(8): 531-535.
41. Huang G, Shagramanova K, Chan S. Formation of odontoblast –like cells from cultured human dental pulp cells on dentin in vitro. J Endod 2006; 32(11): 1066-1073.
42. Wei x. Ling J, Wu L et al. Expression of mineralization markers in dental pulp cells. J Endod 2007; 33(6): 703-8.
43. Zhang W, Walboomers X, Shi S et al. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 2006; 12(10): 2813-23.
44. Batouli S, Miura M, Brahim J et al. Comparison of stem- cell- mediated osteogenesis and dentinogenesis. J Dent Res 2003; 82(12): 976-81.
45. Sloain A, Smith A. Stem cells and the dental pulp: potential roles in dentin regeneration and repair. Oral Dis 2007; 13: 151-7.
46. Huang A, Chen Y, Chan A et al. Isolation and characterization of human dental pulp stem/stromal cells from nonextracted crown fractured teeth requiring root canal therapy. J Endod 2009; 35(5): 673-681.
47. Yu J, Deng Z, Shi J et al. Differentiation of dental pulp stem cells into regular shaped dentin-pulp complex induced by tooth germcell conditioned medium. Tissue Eng 2006; 12(11): 3097-3105.
48. Miura M, Gronthos S, Zhao M et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 2003; 100(10): 5807-5812.
49. Nor JE. Tooth regeneration in operative dentistry. Oper Dent 2006; 31(6): 633-42.
50. Sonoyama W, Liu Y, Fang D et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006; 1: e79.
51. Sonoyama W, Liu Y, Yamaza T et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 2008; 34(2): 166-71.
52. Yalvac M, Rizvanov A, Kilic E et al. Potential role of dental stem cells in the cellular therapy of cerebral ischemia. Current Pharmaceutical Design 2009; 15(33): 3908-3916.
53. Nosratl, Widenfalk J.OIson L et al. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons invitro and rescue motoneurons after spinal cord injury. Dev Biol 2001; 238: 120-132.
54. Stevens A, Zuliani T, Olejnik C et al. Human dental pulp stem cells differentiate into neural crest derived melanocytes and have label-retaining and sphere forming abilities. Stem Cells and Development 2008; 17: 1175-1184.
55. Ryu J, Ko K, Lee J et al. Gangliosides are involved in neural differentiation of human dental pulp- derived stem cells. Biochemical and Biophysical Research 2009; 387(2): 266-271.
56. Ernest W, Saugspier M, Felthaus O et al. Comparison of murine dental follicle precursor and retinal progenitor cells after neural differentiation in vitro. Cell Biology International 2009; 33(7): 758-764.
57. Song J, Stefanik D, Damek-Poprawa M et al. Differentiation and regenerative capacities of human odontoma–derived mesenchymal cells. Differentiation 2009; 77(1): 29-37.
58. Folkman J, Klagsbrun M. Angiogenic factors. Science 1987; 235(4787): 442-7.
59. Ferrara N, Gerber H, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669-76.
60. Keyt B, Nguyen H, Berleau L et al. Identification of vascular endothelial growth factor determinants for binding KDR and Flt-1 receptors: generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem 1996; 271: 5638-46.
61. Artese L, Rubini C, Ferrero G et al. Vascular endothelial growth factor (VEGF) expression in healthy and inflamed human dental pulps. J Endod 2002; 28(1): 20-23.
62. Roberts-Clark DJ, Smith AJ. Angiogenic growth factors in human dentin matrix. Arch Oral Biol 2000; 45(11): 1013-16.
63. Goncalves S. Dong Z, Bramante C et al. Tooth sliced- based models for the study of human dental pulp angiogenesis. J Endod 2007; 33(7): 811-4.
64. Gaitonde PS, Jadhav RM, Dastur RS. Epidermal growth factor receptor and tenascin expression in astrocytic gliomas. Annals of Neurosciences 2005; 12(4): 74-78.
65. Hu C, Zhang C, Qian Q et al. Reparative dentin formation in rat molars after direct pulp capping with growth factors. J Endod 1998; 24(11): 744-51.
66. DenBesten P, Machule D, Gallagher R et al. The effect of TGF-β on dentin apposition and hardness in transgenic mice. Adv Dent Res 2001; 15: 39-41.
67. Tai T, Chan C, Lin C et al. Transforming growth factor 2 regulates growth and differentiation of pulp cells via ALK5/Smad 2/3. J Endod 2008; 34(4): 427-32.
68. Nakashima M, Nagasawa H, Yamada Y et al. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2 and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol 1994; 162(1): 18-28.
69. Saito T, Ogawa M, Hata Y et al. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod 2004; 30(4): 205-208.
70. Nakashima M. Induction of dentin in amputated pulp of the dogs by recombinant bone morphogenetic proteins -2 and -4 with collagen matrix. Arch oral Biol 1994; 39: 1085-9.
71. Tziafas D, Alvanoub A, Papadimitriouc S et al. Effects of recombinant basic fibroblast growth factor, insulin like growth factor-II and transforming growth factor-1 on dog dental pulp cells in vivo. Arch Oral Biol 1998; 43(6): 431-444.
72. Kikuchi N, Kitamura C, Morotomi T et al. Formation of dentin- like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J Endod 2007; 33(10): 1198-1202.
73. Ishimatsu H, Kitamura C, Morotomi T et al. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor -2 from gelatin hydrogels. J Endod 2009; 35(6): 858-865.
74. He J, DahI T, Veis A et al. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res 2003; 44(Suppl 1): 240-5.
75. Srivastava NK, Yadav R and Pradhan S. Metabolic myopathies: clinical, biochemical, genetic and histopathological basis of diagnosis. Annals of Neurosciences 2005; 12(4): 87-98.
76. Nakamura Y, Hammarstrom: L.Matsumoto K et al. The induction of reparative dentin by enamel proteins. Int Endod J 2002; 35(5): 407-17.
77. Hammarstrom: L. Enamel matrix, cementum development and regeneration. J Clin Periodontal 1997; 24(9): 658-68.
78. Sachlos E, Czernuszka J. Making tissue engineering scaffolds work. Review :the application of solid f reef orm fabrication technology to the production of tissue engineering scaffolds. Euro Cell Mater 2003; 5: 29-39.
79. Athanasiou K, Niederauer g, Agrawal C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996; 17(2): 93-102.
80. Taylor M, Daniels A, Andriano K. Six bioabsorbable polymers: In vitro acute toxicity of accumulated degradation products. J Appl Biomater 1994; 5: 151-7.
81. Trojani C, Weiss P, Michiels J et al. Three dimensional culture and differentiation of human osteogenic cells in an Injectable hydroxypropyl-methylcellulose hydrogel. Biomaterials 2005; 26(27): 5509-17.
82. Bohl K, Shon J, Rutherford B et al. Role of synthetic extracellular matrix in development of engineered dental pulp. J of Biomater Sci, Polymer Ed 1998; 9(7): 749-764.
83. Hench L. Bioactive materials: the potential for tissue regeneration. J Biomed Mater Res 1998; 41(4): 511-518.
84. Blaker J, Gough J, Maquet V et al. In vitro evaluation of novel bioactive composites based on Bioglass-filled polylactide foams for bone tissue engineering scaffolds. J Biomed Mater Res 2003; 67(4): 1401-11.
85. Zhang W, Walboomers X, van Kuppevelt T et al. The performance of human dental pulp stem cells on different three dimensional scaffold materials. Biomaterials 2006; 27(33): 5658-68.
86. Gebhardt M, Murray P, Namerow K et al. Cell survival within pulp and periodontal constructs. J Endod 2009; 35(1): 63-66.
87. Raja S, Naidu M. Platelet rich fibrin-evolution of a second generation platelet concentrate. Indian J Dent Res 2008; 19(1): 42-46.
88. Kitasako Y, Shibata S, Pereira P et al. Short term dentin bridging of mechanicallyexposed pulps capped with adhesive resin systems. Oper Dent 2000; 25(3): 155-62.
89. Hargreaves K, Geisler T, Henry M et al. Regeneration potential of the young permanent tooth: what does the future hold? J Endod 2008; 34(7S): S51-S56.
90. Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 2005; 31 (10): 711-8.
91. Gotlieb E, Murray P, Namerow K et al. An ultrastructural investigation of tissue engineered pulp constructs implanted within endodontically treated teeth. JADA 2008; 139(4): 457-465.
92. Huang G, Sonoyama W, Liu Y et al. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J Endod 2008; 34(6): 645-651.
93. Chan C, Lan W, Chang M et al. Effects of TGF- betas on the growth, collagen synthesis and collagen lattice contraction of human dental pulp fibroblasts in vitro. Arch Oral Biol 2005; 50(5): 469-79.
94. Hayashi K, Kubo T, Doi K et al. Development of new drug delivery system for implant bone augmentation using a fibroblast growth factor-gelatin hydrogel complex. Dent Mater J 2007; 26(2): 170-7.
95. Tonomura A, Sumita Y, Ando Y et al. Differential inducibility of human and porcine dental pulp- derived cells into odontoblasts. Connective Tissue Res 2007; 48(5): 229-238.
96. Burdick J, Ansetn K. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002; 23(22): 4315-4323.
97. Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng 2004; 10(9-10): 1316-22.
98. Huang G, Yamaza T, Shea L et al. Stem /progenitor cell – mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A (ahead In Print) doi: 10.1089/ten. Tea. 2009.0518.
99. Adhirajan N, Shanmugasundaram N, Shanmuganthan S et al. Functionally modified gelatin microspheres impregnated collagen scaffold as novel wound dressing to attenuate the proteases and bacterial growth. Eur J Pharm Sci 2009; 36(2-3): 235-45.
100. Edwards P, Mason J. Gene enhanced tissue engineering for dental hard tissue regeneration : (1) overview and practical considerations. Head Face Med 2006; 2: 12.
101. Alden T, Varady P, Kallmes D et al. Bone morphogenetic protein gene therapy. Spine 2002; 27: S87-93.
102. Rutherford RB. BMP-7 gene transfer to inflamed ferret dental pulps. Eur J Oral Sci 2001; 109(6): 422-424.
103. Yang X, van der Kraan P, Bian Z et al. Mineralized tissue formation by BMP2-transfected pulp stem cells. J Dent Res 2009; 88(11): 1020-1025.
104. Thibodeau B,Teixeira F.Yamauchi M et al. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod 2007; 33(6): 680-689.
105. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of literature. Pediatr Dent 2007; 29(1): 47-50.
106. Shah N, Logani A, Bhaskar U et al. Efficacy of revascularization to induce apexification / apexogenesis in infected, nonvital, immature teeth: a pilot clinical study. J Endod 2008; 34(8): 919-925.
107. Jung I, Lee S, Hargreaves K. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod 2008; 34(7): 876- 887.
108. Ring K, Murray P, Namerow K et al. The comparison of the effect of endodontic Irrigation on cell adherence to root canal dentin. J Endod 2008; 34 (12): 1474-9.
109. Sato T, Hoshino E, Uematsu H et al. In vitro antimicrobial susceptibility to combinations of drugs on bacteria from carious and endodontic lesions of human deciduous teeth. Oral Microbiol Immunol 1993; 8: 172-6.
110. Terranova V, Odziemiec C, Tweden K et al. Repopulation of dentin surfaces by periodontal ligament cells and endothelial cells, effect of basic fibroblast growth factor. J Periodontol 1989; 60: 293-301.
111. Marx RE. Platelet rich plasma: evidence to support its use. J Oral Maxillofacial Surg 2004; 62(4): 489-496.
112. Wang X, Thibodeau B, Trope M et al. Histologic characterization of regenerated tissues in canal space after the revitalization / revascularization procedure of immature dog teeth with apical periodontitis. J Endod 2010; 36(1): 56-63.
113. Okamoto Y, Sonoyama w, Ono M et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod 2009; 35(3): 367-72.
114. Matsui S, Takahashi C, Tsujimoto Y. Stimulatory effects of low–concentration reactive oxygen species on calcification ability of human dental pulp cells. J Endod 2009; 35(1): 67-72.
115. Ando Y, Honda M, Ohshima H et al. The Induction of dentin bridge- like structures by constructs of subcultured dental pulp-derived cells and porous HA/TCP in porcine teeth. Nagoya J Med Sci 2009; 71 (1-2): 51-62.
116. Matsuzaka K, Muramatsu T, Katakura A et al. Changes in homeostatic mechanism of dental pulp with age: expression of the core binding factor alpha-1, dentin sialoprotein, vascular endothelial growth factor, and heat shock protein 27 messenger RNAs. J Endod 2008; 34(7): 818-821.