Annals of Neurosciences, Vol 12, No 4 (2005)
Annals of Neurosciences, Volume 12, Issue 4 (October), 2005
AN UPDATE ON THE ROLE OF ENVIRONMENTAL FACTORS IN PARKINSON'S DISEASE
Corresponding author
Dr. M. P. Singh
Industrial Toxicology Research Centre (ITRC)
Mahatma Gandhi Marg Post Box-80
Lucknow- 226 001, UP, India
Tel No: +91-522-2613618 Ext. 337
Fax: +91-522-2628227
E-mail:
Abstract
Parkinson's disease (PD) is an idiopathic, progressive and degenerative disorder of the central nervous system, characterized by slow and reduced movement, muscular rigidity, tremor and postural abnormality. It is second most common neurodegenerative disorder with the prevalence of 1–2 percent after the age of 50 years. PD is a multi-factorial disease, caused by the combination of age, genetic and environmental factors. Environmental exposures to pesticides, herbicides and heavy metals play a critical role in the onset and progression of PD. The susceptibility of individuals for PD depends on the metabolic enzymes involved in the detoxification of selected environmental chemicals and toxicants. Inherited differences in metabolic enzymes are mainly responsible for the neuronal degeneration. Genetic polymorphisms in the toxicant responsive genes greatly alter the susceptibility of chemically induced PD. Evidences obtained from genome wide analysis and biochemical, molecular and epidemiological studies have concluded the significant contribution of environmental factors in the onset of PD. In this article, an update on the role of environmental factors in the onset and progression of Parkinson's disease has been discussed.
Key Words: Parkinson's disease (PD), Environmental Factors, Toxicants, Metals and Pesticides
Introduction
Parkinson's disease (PD) is named after British physician James Parkinson who gave the first cogent description of the syndrome in 1817. Marshall Hall in 1941 referred PD as paralysis agitans. Idiopathic PD is characterized by the progressive degeneration of dopamine-producing cells in the substantia nigra pars compacta region of the mid brain. The main phenotypic symptoms of PD are trembling in hands, legs, jaws and face, stiffness in the limbs and trunk, slowness of the involuntary and emotional movements and postural abnormality. PD is second most common neurodegenerative disorder after Alzheimer's disease. It seems to develop from the interaction of the inherited genetic susceptibility, environmental exposures and age of an individual. Role of environmental factors has acquired attention only when the use of a synthetic heroin was found to elicit severe Parkinsonism in addicts. Following this discovery, an initiating agent l-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine (MPTP) was identified as contaminant in synthetic heroin. Since, MPTP possess structural similarities with some herbicides and pesticides, therefore, the involvement of these pesticides and herbicides in the onset of PD has been predicted. During the last two decades very limited studies have been conducted worldwide on the identification of various environmental factors responsible for the onset of PD, however, studies have also shown increased incidence of PD associated with exposure to pesticides, heavy metals, solvents and viral infections such as encephalitis and head injuries. Advancement in molecular biology has already shown clear-cut involvement of environmental chemicals and toxicants in PD. In the present article, the role of environmental factors in the onset and progression of Parkinson's disease has been discussed along with the evidences obtained from epidemiological, biochemiccil and molecular studies.
Prevalence
PD is a common disease in the aged individuals that affects about 0.4% of the population over the age of 50 years and 1% of the population over the age of 65 years (1–4). Prevalence of PD has been reported up to 280 individuals per million in North America, 59 individuals per million in Africa, 44 individuals per million in China, 19 individuals per million in India and 150 individuals per million in Western Europe (1–4). In the North America and Western Europe the incidence may reach up to 1 individual per 40 individuals in the aged population (4). Parsis are the most affected population in India and up to 328 cases per million have been reported. However, the incidence of the disease begins at the age of 50 years but the severity of the disease may increase throughout the life (5, 6). PD is more common in men than in women (7) and seems to be more widespread in northern countries than the southern ones; however, reports are also available in literature mentioning the lack of gender preference (8).
Modifying Factors
PD is a multi-factorial disease and contributed by a combination of age, genetic and environmental factors (9–11). PD is not a disease of childhood; however, some cases of the disease have been found to be associated with exposures to environmental toxicants throughout life, beginning in the childhood or in utero (12). Many researchers believe that several modifying factors in combination are involved in the manifestation of PD that include Caucasian ancestry (13), environmental toxins, herbicide/pesticide exposure (14–16), rural residence, metal exposure (17–23), higher intake of dietary fats, genetic predisposition, free radicals, accelerated aging, male gender (7), conservative pre-Parkinson's personality and family history. Many investigators have reported the involvement of both chemical toxins and genetic mutations (24–27). Scientists are actively involved from the last several decades, in unraveling the molecular mechanism of the death of brain cells and ultimately the rigidity, tremors and other symptoms of PD. The toxic effects resulting from the oxidative stresscombined with environmental exposures could act synergistically to cause PD (28). PD has been associated with drinking well-water, farming, and the exposure to certain wood preservatives (29, 30, 31, 32, 33, 34). The late onset and slow-progressing nature of PD has prompted the consideration of environmental exposure to agrochemicals, including pesticides, as a risk factor (35). It is also reported that head trauma and several neurotoxicants, including MPTP paraquat, dieldrin, manganese (Mn) and salsolinol may lead to PD (36). The consumption of Cycas circinalis seed in the Japanese population has been found to induce PD like syndrome (37). Single nucleotide polymorphisms (SNPs) in the detoxification genes determine the susceptibility of an individual towards the onset of a disease. SNPs in cytochrome P-450 (CYP), glutathione S-transferase (GST), paraoxanase (PON), nitric oxide synthase (NOS) and several other genes have been extensively studied in many populations and have been correlated with the onset of PD. Genetic factors mainly contribute in the early onset of the disease; however, several studies of twin pairs have shown that genetic factors do not play a critical role in PD (38) until the environmental factors acts on the genetically susceptible individuals.
Environmental Factors
Parkinson's disease can originate from long-term exposure of the central nervous system to environmental chemicals. It is also reported that the workers exposed to industrial solvents might develop PD. It is reported that individuals with familial PD possesses much lover level of detoxifying enzymes than healthy one and accumulation of toxic chemicals is higher in such individuals compared with unexposed one. Heavy metals help in catalyzing free radical reactions that destroy the dopamine-producing cells and have therefore been implicated as the causative agents in the onset and progression of PD (15, 17–21, 23). Individuals exposed to high environmental levels of Mn, which include miners, welders, and those living near ferroalloy processing plants, display a syndrome known as manganism, that is best characterized by debilitating symptoms resembling those of PD (39). Manganese decreases monoamine oxidase (MAO) activity and inhibits the respiratory chain that accumulates in mitochondria and inhibits efflux of calcium (40). The essential metal iron has long been implicated in the neuronal damage associated with PD due to dopamine and motor disturbances (41). Populations exposed to aluminum contamination in the drinking water have high risk of developing PD (42, 43). Aluminium facilitates the passage of toxic chemicals into the brain; however, the brain is normally protected from undesirable chemicals by the blood-brain barrier (44). Aluminum has neurotoxic properties and inhibits the synthesis of important brain chemicals that has the potential to block nucleic acid metabolism within nerve cells. Aluminium interferes with magnesium in the regulation of neurotransmitter receptors (45). Aluminum overload induces clinical symptoms of Encephalopathy (46). Many researchers agree that aluminum is an important factor in dementia and have also demonstrated a positive relationship between aluminum in drinking water and neurological conditions. A unique occurrence of Parkinson's disease in the indigenous population of Guam in the Western Pacific has been reported where the soil and drinking water have high levels of aluminum and low levels of magnesium and calcium (47).
Accumulation of heavy metals, such as cadmium, lead and mercury are known to deteriorate the brain. Mercury is considered to be the most hazardous metal to the brain (48). Organic mercury compounds have been reported to destroy the nerve cells and resulting tremors. A link between dental amalgam and PD has been reported and the amalgam dental material in tooth fillings have been projected as the major source of the introduction of mercury into the bloodstream (49). The essential metal iron has long been implicated in the neuronal damage associated with dopamine and motor disturbances (41). Iron overload is known to aggravate Parkinson's disease as evidenced by the presence of high levels of iron in the substantia nigra (50). Iron tends to catalyze free-radical reactions that destroy dopamine-producing cells (21). Copper levels have been reported to be significantly higher in the cerebrospinal fluid of PD patients and the higher level is probably due to copper dependent enzyme required to convert tyrosine into levodopa. Undesirable changes in the composition of the intestinal flora and the resulting overgrowth in small intestine are known to induce PD (51). Drugs and allergic inflammation of the intestinal wall allow partly digested food fragments and bacterial endotoxins to reach into the brain and act as neurotoxins (52).
Epidemiological, Biochemical and Molecular Approaches of PD and Environmental Factors
In the last few decades, the environmental theory on the etiology of Parkinson disease has acquired significant importance. From an experimental point of view, several new models of PD induced by pesticides have been proposed. In vitro studies have also provided clues that several pesticides stimulate the formation of alpha-synuclein fibrils. A meta-analysis of all case-control studies so far performed have shown a positive, statistically significant association between pesticide exposure and PD (53). Epidemiological studies have identified exposure to agricultural chemicals as potential environmental risk factors for PD (30, 42) in aged individuals and cannot be explained by genetic factors (54). Variation in PD mortality by geographical regions, as reported in several countries, has also been found consistent with an environmental exposure etiology (55–57). It is reported that chronic exposure to a common pesticide can reproduce the anatomical, neurochemical, behavioral and neuropathological features of PD. The use of a synthetic heroin called MPTP (1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine) causes severe, irreversible Parkinsonism, a syndrome, closely related to PD (37). These examples have shown that a PD-like disorder can be caused directly by a chemical in the environment. Chronic subcutaneous exposure to low doses of rotenone causes highly selective nigrostriatal dopaminergic lesions. Dithiocarbamate pesticides potentiate the toxicity of both 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine and paraquat in mouse models of PD and several studies have suggested selective dithiocarbamates mayalter the kinetics of different endogenous and exogenous compounds and enhance their neurotoxicity. The herbicide N, N'-dimethyl-4, 4'-bipyridylium (paraquat) acts as a modifying factor in the onset of PD due to its structural similarity to MPP + (1-methyl-4-phenylpyridinium ion), (58). Higher paraquat (PQ) levels are evident at 24 h after its administration thereby indicating its involvement in brain toxicity (59). Direct injection of paraquat into brain alters dopamine level, personal behavior and induces neuronal loss (60–62), however, systemic administration does not yield consistent evidence of neurotoxicity in rodents (61, 63, 64). Repeated intraperitoneal paraquat injections killed dopaminergic neurons in the substantia nigra (SN) pars compacta, as assessed by the dose- and age-dependent stereological counting of tyrosine hydroxylase (TH)-immunoreactive, Nissl-stained neurons and the cell loss (65). Several lines of evidence have indicated selective vulnerability of dopaminergic neurons to paraquat (65). The numbers of GABAergic cells are reported to remain unchanged in the SN pars reticulata and counting of Nissl-stained neurons in the hippocampus did not reveal any change in paraquat-treated mice (65). Degenerating cell bodies have been observed by silver staining in the SN pars compacta, and glial response has been known to present in the ventral mesencephalon but not in the frontal cortex and cerebellum (65) following paraquat administration however, no significant depletion of striatal dopamine has been reported (65). On the other hand, enhanced dopamine synthesis has been suggested by an increase in TH activity (65). These findings unequivocally showed selective dopaminergic degeneration, one of the pathological hallmarks of PD. The apparent discrepancy between neurodegenerative and neurochemical effects represents an important feature of the paraquat model and is probably a reflection of compensatory mechanisms by which neurons that survive damage, are capable of restoring neurotransmitter tissue levels (65).
Dithiocarbamate fungicides also possess potent dopaminergic activity and diethyldithiocarbamates, a class of dithiocarbamates, augmented the neurotoxicity of MPTP (66, 67). Several studies have suggested that dithiocarbamates alter the kinetics of different endogenous and exogenous compounds thereby enhancing their neurotoxicity (68). Acute intraperitoneal administration of manganese ethylenebisdithiocarbamate (maneb or MB) exacerbated attenuation in locomotor activity and augmentation in catalepsy produced by MPTP in mice. Maneb itself inhibited locomotor activity and augments aloperidol-induced catatonia (68, 69). Neurological impairments resembling those of PD have also been reported in individuals exposed to MB (70, 71). Residual levels of organochlorine pesticide dieldrin have been found in brains of one third of the PD patients as compared with controls (72, 73). Mancozeb (manganese-zinc-ethylenebisdithiocarbamate) andzineb (zinc-ethylenebisdithiocarbamate) dose-dependently attenuate high affinity dopamine uptake and tyrosine hydroxylase (TH) positive neurons and increase cytotoxicity in mesencephalic striatal primary co-cultures (72). Triadimefon, a triazole fungicide blocks dopamine transporter and produces behavioral effects resembling those of cocaine and d-amphetamine (74–78).
The combined pesticide exposures may act as important environmental risk factor for Parkinsonism (79). Paraquat (PQ) and matneb (MB) exposure during critical periods of development could permanently change the nigrostriatal dopamine (DA) system and enhance its vulnerability to subsequent neurotoxicant challenges (67). These findings indicate that exposure to pesticides during the prenatal period can produce permanent and progressive lesions of the nigrostriatal dopaminergic system. An enhanced adult susceptibility to these pesticides has suggested that developmental exposure to neurotoxicants may be involved in the induction of neurodegenerative disorders (67); however, such pesticide exposure is irrelevant for medico-legal considerations (66). A consistent pattern of high Parkinson's disease morbidity has been found among occupational groups employed in agriculture and horticulture in Denmark (80). Although, the risk of PD is proportional to pesticide exposure time, no significant dose-response relationship has yet been established (53) and no specific group of pesticide has been identified. PD is also associated with a systemic defect in mitochondrial complex I activity (81) by the environmental toxicants. Animal models have also shown that exposure to the inhibitors of mitochondrial complex I including pesticides, is sufficient to reproduce the features of PD. Complex I defects result in oxidative stress that increases the susceptibility of neurons to excitotoxic death (28). Betarbet and his associates (82) have reported that chronic and systemic inhibition of mitochondrial complex I enzyme by the lipophilic pesticide rotenone, causes highly selective nigrostriatal dopaminergic degeneration and associated behaviorally with hypokinesia and rigidity. Nigral neurons in rotenone-treated rats accumulate fibrillar cytoplasmic inclusions that contain ubiquitin and alpha-synuclein (82). Striatal neurons containing DARPP-32 (dopamine and cAMP-regulated phosphoprotein) and neurons of the globus pallidus and subthalamic nucleus remain intact with normal morphology following rotenone treatment (83).
Gene Expression Based Approaches of PD and Environmental Factors
Recent developments in molecular technologies have made it possible to study and identify the mechanisms of neurodegeneration using DNA microarrays, those were not feasible with conventional biochemical procedures. Microarray based analysis has also provided a number of evidences for the involvement of environmental factors in the onset of PD. The array data of 6-OHDA mediated PD are already available that have suggested that dopamine (DA) denervation of the striatum results in an impairment in the DA-protein kinase A-cyclin dependent kinase 5-protein phosphatases cascade that regulates the state of phosphorylation and activity of DARPP-32 (84). Most of the gene alterations have been reversed by Dl and D2 receptor antagonist R-apomorphine that prevents MPTP induced neurotoxicity in mice (85). An interesting finding has also been reported in which 6-OHDA induced denervation resulted in modulation of the expression of genes encoding for components of dopamine signaling network. PKA and CD5 are known to regulate DARPP-32, a mediator of dopamine signaling, by phosphorylating specific sites at thr-34 and thr-75. A target of PKA-induced thr-34 phosphorylated DARPP-32 has been reportedas protein phosphatase PP1 (86) and PP2A that play a pivotal role in the dephosphorylation of phospho-DARPP-32 at the thr-75 site and denervation modulates the expression of genes that target DARPP-32 as an integration crossroad for intracellular dopamine signaling. A dose-dependent protective effect of PD in coffee and tea drinkers and smokers in an ethnic Chinese population is reported (87). Dopamine (DA), R-apomorphine (R-APO), polyphenol (-)-epigallocatechine-3-gallate (EGCG) and melatonin have also been reported as neuroprotective and free radical scavengers in PD patients (88). A concentration and time-dependent correlation between R-APO, DA, EGCG, and melatonin in modulation of cell survival/cell death-related gene pathways has also been confirmed by quantitative real-time PCR and protein profiles.
The cDNA microarray gene expression has indicated that the mechanism of neurodegeneration by MPTP (N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) and 6-hydroxydopamine is a complex cascade of vicious circles (89). Alteration of genes associated with iron metabolism has supported oxidative stress induced neurodegeneration and iron deposition in substantia nigra pars compacta (SNPC) (89). MPTP and R-apomorphine induced alterations in 49 different genes involved in oxidative stress and inflammation (90). It may be hypothesized that an additional cascade might act in parallel to oxidative stress and inflammation to converge into a common pathway leading to neurodegeneration. R-apomorphine is known to prevent the over expression of several genes participating in cell death (90). The concept has been supported by recent complementary DNA (cDNA) microarray gene expression studies, which show the existence of a gene cascade of events occurring in the nigrostriatal pathway of MPTR 6-OHDA and methamphetamine animal models of PD (91). Genetic correlates of the alterations produced by 6-hydroxydopamine-induced denervation in the nucleus of striatum (92) have shown the modulation of 50 different genes involved in various cellular functions. In particular, products of the genes modulated by this experimental manipulation are involved both in the intracellular transduction of dopamine signal and in the regulation of glutamate transmission in striatal neurons, providing some information on the possible neuronal events which lead to the reorganization of glutamate transmission in the striatum of Parkinsonian rats (92). It is also possible to develop effective neuroprotective drugs using microarray technology (84, 85, 88, 93–95).
Toxicant Responsive Gene Polymorphism Based Approaches of PD and Environmental Factors
After the human genome sequencing, the polymorphism in the genes have shown an important clue to verify the involvement of toxicant responsive genes in the onset of PD. SNP based studies have shown many evidences of the contribution of environmental factors in the onset of the disease. Many enzymes including the cytochrome P-450 system, the flavin monooxygenase system, esterases, paraoxonases and glutathione transferases metabolize pesticides and are involved in the metabolism of pesticides leading to either activation or inactivation (96, 97). Polymorphism in enzymes would be expected to alter the risk of PD by influencing the deposition of pesticides. Genetic susceptibility to neurotoxins has been reported as one of the major causes of PD and polymorphisms in various forms of cytochrome P-450 genes such ris CYP1A2, CYP2C9, CYP2C19, CYP2E1, CYP2D6 and CYP3A4 have been extensively studied (98). CYP1A1 gene polymorphisms have been found as a genetic susceptible factor for early-onset of PD, however, CYP2E1 Rsal and PstI polymorphisms are not found to be genetic susceptible factors for both early- and late-onset of PD in the Chinese population (99). CYP2D6 is involved in the detoxification of environmental chemicals and toxicants (97, 100). A significant association between the polymorphisms of this gene and PD has already been reported in Australian population (14). A significant association of PD has been found with drugs and toxins metabolism regulatory gene CYP2D6 (98) in several studies; however, association results of these studies have been inconsistent (98). Genotyping of cytochrome P-450 genes such as CYP2D6 (101), CYP3A4, CYP2C9, and NAD (P) H: ubiquinone oxido-reductase gene (NQOl) indicated their involvement in chemically induced PD and helped in understanding the mechanism of pathogenesis of PD. Deprenyl metabolizing cytochrome P450 system in monkey (Cercopithecus aethiops) liver microsomes has been recently reported (102). Genetic polymorphism of the CYP2E1 gene and susceptibility to PD in Taiwanese population was not found to be significantly related (103). A role of environmental factors in the frequency of CYP2D6-deficient alleles in the patients with PD in European population has also been suggested (8). Investigators have also supported the hypothesis that the CYP2D6 gene is not a major gene responsible for PD. The association of CYP2D6*4 allele was not found with earlier PD onset; however, its association with survival (98) was projected. Remarkable and significant genetic polymorphisms in CYP2D6 genes in Indian population have been reported (99, 104), however, evidences are needed to support their role in the onset and progression of PD. Association between polymorphism in the pi class glutathione transferase and PD in the subjects reported with pesticides exposure is of particular interest because diminished glutathione in the substantia nigra is an early finding in PD (105, 106, 107). An association between PON1 gene and PD has been reported in Asians (108); however, in Caucasians no significant association was found in PD and PON1 (109). PON1 gene is involved in the metabolism of oxidized lipids and plays a major role in the metabolism and detoxification of insecticides processed through the cytochrome P450/PON1 pathway (110). Studies conducted by various investigators have also demonstrated population specific allele frequencies in PON1 gene (111) and its association with the onset of PD. Association between polymorphisms in NOS genes and PD in a community based case control study have also elucidated the role of genetic polymorphisms in PD (112).
Conclusion
PD is a multi-factorial disease and caused by age, genetic and environmental factors. Epidemiologcal, biochemical and molecular studies conducted worldwide have shown significant contribution of environmental factors in the onset of PD. Age isa consistent risk factor of PD, however, an age-dependent cumulative insult of toxicants on the genetically susceptible individuals have been found to be mainly responsible for the selective degeneration of nigrostriatal dopaminergic neurons.
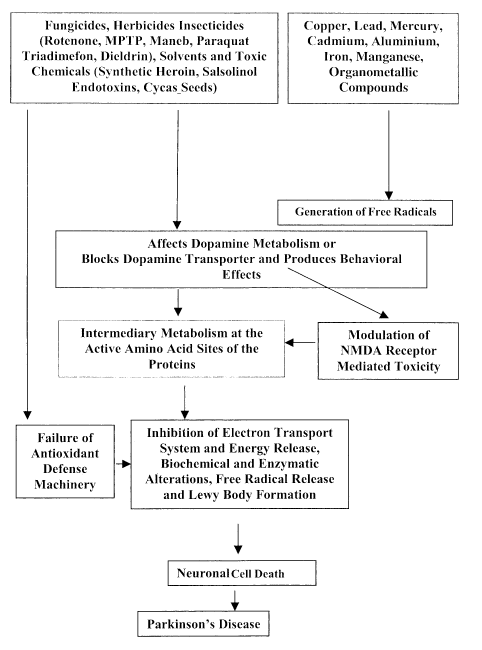
Fig. 1. A diagrammatic representation on the role of environmental factors in the onset and progression of Parkinson's disease. Environmental factors either modulate the antioxidant defense system or generate free radicals or reduce dopamine metabolism and block dopamine transporter. Modulation in any of these parameters may block energy release and thereby induce neuronal cell death in the susbstantia nigra pars compacta region of the mid brain.
References
1. Trenkwalder C, Schwartz J, Gebhard J, Ruland D, Trenkwalder P, Hense HW, Oertel WH. Stanberg trial on epidemiology of Parkinsonism and hypertension in the elderly. Prevalence of Parkinson's disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995; 52:142: 820–827.
2. Fall PA, Axelson O, Fredriksson M, Flansson G, Lindvall B, Olsson JE, Granerus AK. Age-standardized incidence and prevalence of Parkinson's disease in a Swedish community. J Clin Fpidemiol 1996; 49: 637–41.
3. Errea JM, Ara JR, Aibar C, de Pedro-Cuesta J. Prevalence of Parkinson's disease in lower Aragon, Spain. Mov Disord 1999; 14: 596–604.
4. Tanner CM and Ben-Shlomo Y. Epidemiology of Parkinson's disease. Adv Neurol 1999; 80: 152–9.
5. Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med 334: 71–76.
6. Checkoway H and Nelson L. Epidemiologic Approaches to the Study of Parkinson's Disease Etiology Epidemiology 1999; 10: 327–336.
7. Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson's disease in Indian population. J Neurol Sci 2001, 190; 1–2: 49–55.
8. Gerard N, Panserat S, Lucotte G. Roles of gender, age at onset and environmental risk in the frequency of CYP2D6-deficient alleles in patients with Parkinson's disease. Eur Neurol 2002; 48(2): 114–5.
9. Youdim MB, Riederer P. Understanding Parkinson's disease. Sci Am 1997; 276: 52–59.
10. Veidman BA, Wijn AM, Knoers N, Pramotra R Florstink NWIM. Genetic and environmental risk factors in Parkinson's disease. Clin Neurol Neurosurg 1998; 100(1): 15–26.
11. Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson's disease. Neurotoxicology 2002; 23 (4–5): 487–502.
12. Clane DB, Eisen A, McGeer E, Spencer R Alzheimer's disease, Parkinson's disease, and motoneurone disease: abiotrophic interaction between ageing and environment? Lancet 1986; 2: 1067–70.
13. Chaudhury KK, Hu MT, Brooks DJ. Atypical Parkinsonism in Afro-Caribbean and Indian origin immigrants to the UK. Mol Disord 2000, 15; 1: 18–23.
14. Le Couteur DG, McLean AJ, Taylor MC, Woodham BL, Board PG. Pesticides and Parkinson's disease. Biomed & Pharmacother 1999; 53: 122–130.
15. Wesseling C, van Wendel de Joode B, Ruepert C, Leon C, Monge R Hermosillo H, Partanen TJ. Paraquat in developing countries. Int J Occup Environ Health 2001; 7(4): 275–86.
16. Petrovitch H, Ross CW, Abbott RD, Sanderson WT, Sharp DS, Tanner CM, Masaki KH, Blanchettee PL, Popper JS, Foley D, Launer L, White LR. Plantation work and risk factor of Parkinson's disease in a population based longitudinal study. Arch Neurol 2002; 59 (11): 1787–1792.
17. Sziraki I, Mohankumar KP, Rauhala P, Kim HG, Yeh KJ, Chiueh CC. Manganese: a transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced model of Parkinsonism. Neuroscience 1998; 85(4): 1101–1111.
18. Yantiri R Andersen JK. The role of iron in Parkinson's disease and 1-methyl 4-phenyl 1,2,3,6, tetra hydropyridine toxicity. IUBMB Life 1999; 48 (2): 139–141.
19. Biernat H, Ellias SA, Wermuth L, Cleary D, de Olivlera Santos EC, Jorgensen PJ, Feldman RG, Grandjean R Tremor frequency patterns in mercury vapour exposure compared with early Parkinson's disease and essential tremor. Neuro toxicology 1999, 20; 6: 945–952.
20. Aschner M. Manganese: Brain transport and emerging research needs. Environ Health Perspect 2000; 108 Suppl 3: 429–432.
21. Linert W, Jameson GN. Redox reactions of neurotransmitters possibly involved in the progression of Parkinson's disease. J Inorg Biochem 2000; 79 (1–4): 319–326.
22. Sayre LM, Perry G, Atwood CS, Smith MA. The role of metal in neurodegerative disease. Cell Mol Biol (Noisy-de grand) 2000; 46(4): 731–741.
23. Floor E. Iron as a vulnerability factor in nigrostriatal degeration in aging and Parkinson's disease. Cell Mol Biol (Noisy-de grand) 2000; 46 (4): 709–720.
24. Allan W. Inheritance of the shaking palsy. Arch Int Med 1987; 60: 424–436.
25. Sveinbjornsdottir S, Hicks AA, Jonsson T, Petursson H, Gugmundsson G, Frigge ML, Kong A, Gulcher JR, Stefansson K. Familial aggregation of Parkinson's disease in Iceland. N Fng J Med 2000; 343: 1765–1770.
26. Scott WK, Nance MA, Watts RL, Hubble JP, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH Jr, Goetz CG, Small GW, Masterman D, Mastaglia F, Laing NG, Stajich JM, Slotterbeck B, Booze MW, Ribble RC, Rampersaud E, West SG, Gibson RA, Middleton LT, Roses AD, Haines JL, Scott BL, Vance JM, Pericak-Vance MA. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA 2001; 286: 2239–2244.
27. McNaught K St P, Beliazaire R, Isacson O, Jenner P, Olanow CW. Altered proteosomal function in sporadic Parkinson's disease. Exp Neurol 2003; 179 (1): 38–46.
28. Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson's disease. Neuroscientist 2002; 8 (3): 192–7.
29. Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Envi Res 1997; 73: 92–100.
30. Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson's disease. Neurology 1993; 43: 1693–7.
31. Seidler A, Hellenbrand W, Robra BR Vieregge R Nischan R Joerg J, Oertel WH, Ulm G, Schneider E. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology 1996; 46: 1275–84.
32. Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology 1997; 48: 1583–8.
33. Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for Parkinson's disease in the Emilia-Romagna region of Italy. Neurotoxicology 1998; 19: 709–12.
34. McCann SJ, LeCouteur DG, Green AC, Brayne C, Johnson AG, Chan D, McManus ME, Pond SM. The epidemiology of Parkinson's disease in an Australian population. Neuroepidemiology 1988; 17: 310–7.
35. Tsai CH, Lo SK, See EC, Chen HZ, Chen RS, Weng YH, Chang FC, Lu CS. Environmental risk factors of young onset Parkinson's disease: a case-control study. Clin Neurol Neurosurg 2002; 104 (4): 328–33.
36. Chun HS, Gibson GE, DeGiorgio LA, Zhang H, Kidd VJ, Son JH. Dopaminergic cell death induced by MPP(+), oxidant and specific neurotoxicants shares the common molecular mechanism. J Neurochem 2001, 76; 4: 1010–21.
37. Spencer PS. Guam ALS/Parkinsonism-Dementia: A long latency disorder caused by "slow toxin(s)" in food? Can J Neurol Sci 1987; 14:347–357.
38. Tanner CM, Ottman R, Goldman SM, EHenberg J, Chan R Mayeaux R, Langston JW. Parkinson Disease in twins: An etiologic study. JAMA. 1999; 281: 341–346.
39. Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol 2003; 66 (1): 1–13.
40. Zhang S, Zhou Z, Fu J. Effect of manganese chloride exposure on liverand brain mitochondria function in rats. Environ Res 2003; 93 (2): 149–5.
41. Gorell JM, Johnson CC, Rubicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson's disease. Neurotoxicology 1999; 20 (2–3): 239–247.
42. Gorell GM, Johnson CC, Rybicki BA, Peterson EL and Richardson RJ. The risk of Parkinson's disease with exposure to pesticides, farming, well water, and rural living. Neurology 1998; 50: 1346–1350.
43. Altschuler E. Aluminium containing antacids as a cause of idiopcithic Parkinson's disease. Med Hypothesis 1999. 53; 1: 22–23.
44. Glanz J. Hemoglobin reveals new role as blood pressure regulator. Science 1996; 271(5256): 1670.
45. Goyer RA. Toxic and essential metal interactions. Annu Rev Nutr 1997; 17: 37–50.
46. Luda E. The EEG in progressive dialysis encephalopathy: the EEG in diagnosing and screening for PDE. (Part. I). Ital J Neurol Sci. 1984; 5(4): 369–73.
47. Armon C. Daube JR, O'Brien PC, Kurland LT, Mulder DW. When is an apparent excess of neurologic cases epidemiologically significant? Neurology 1991, 41; 11: 1713–8.
48. Elwan KB, Pamphlett R. Increcised inorganic mercury in spinal motor neurons following chelating agents. Neurotoxicology 1996; 17(2): 343–9.
49. Willershausen-Zonnchen B, Zimmermann M, Defregger A, Schramel R Hamm G. Mercury concentration in the mouth mucosa of patients with amalgam fillings Dtsch Med Wochenschr 1992; 117(46): 1743–7.
50. Youdim MB, Ben-Shachar D, Riederer P. Iron in brain function and dysfunction with emphasis on Parkinson's disease. Eur Neurol 1991; 31 Suppl 1: 34–40.
51. Bergogne Berezin E. (1985). Microbial ecology of the colon. Ann Gastroenterol Hepatol (Paris) 1985, 21; 6: 383–388.
52. SchoeffelGJ. The apicoectomy myth: failures need surgery. Dent Today 1989; 8(2):40-l.
53. Priyadarshi A, Khuder SA, Schaub EiA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology 2000; 21 (4): 435–40.
54. Tanner CM, Chen B, Wang WZ, Peng ML, Liu ZL, Liang XL, Kao LC, Gilley DW, Schoenberg BS. Environmental factors in the etiology of Parkinson's disease. Can J Neurol Sci 1987; 14: 419–423.
55. Svenson LW, Piatt GLI, Woodhead SE. Geographic variations in the prevalence rates of Parkinson's disease in Alberta. Can J Neurol Sci 1993; 20: 307–311.
56. Imaizumi Y Geographical variations in mortality from Parkinson's disease in Japan, 1977–1985. Acta Neurol Scand 1995; 91: 311–316.
57. Lanska DJ. The geographic distribution of Parkinson's disease mortality in the United States. J Neurol Sci 1997; 50: 63–70.
58. Langston JW, Ballard PA, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983; 219: 979–980.
59. Widdowson PS, Farnworth MJ, Simpson MG, Lock EA. Influence on age on the passage of paraquat through the blood-brain barrier in rats: A distribution and pathological examination. Hum Exper Toxicol 1996; 15: 231–236.
60. Calo M, lannone M, Passafaro M, Nistico G. Selective vulnerability of hippocampal CA3 neurones after microinfusion of paraquat into the rat substantia nigra or into the ventral tegmental area. J Comparative Pathol 1990; 103: 73–78.
61. Bagetta G, Corasaniti MI, lannone M, Nistico G, Stephenson JD. Production of limbic motor seizures and brain damage by systemic and intracerebral injections of paraquat in rats. Pharmacol Toxicol 1992; 71: 443–448. '
62. Liou HH, Chen RC, Tsai YF, Chen WP, Chang YC, Tsai MC. Effects of paraquat on the substantia nigra of the Wistar rats: Neurochemical, histological, and behavioral studies. Toxicol Appl Pharmacol 1996; 137: 34–41.
63. Markey SP Weisz A, Bacon JP. Reduced paraquat does not exhibit MPTP-like neurotoxicity. J Anal Toxicol 1986; 10 (6): 257.
64. Perry TL, Yong VW, Wall RA, Jones K. Paraquat and two endogenous analogues of the neurotoxic substance N-methyl-4-phenyl-l, 2, 3, 6-tetrahydropyridine do not damage dopaminergic nigrostriatal neurons in the mouse. Neurosci Lett 1986; 69: 285–289.
65. McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002; 10 (2): 119–27.
66. Vieregge P. Pesticide exposure and Parkinson's syndrome-the epidemiological and experimental evidence. Nervenarzt 2002; 73(10): 982–9.
67. Thiruchelvam M, Richfield EK, Goodman BM, Baggs RB, Cory-Slechta DA. Developmental exposure to the pesticides paraquat and maneb and the Parkinson's disease phenotype. Neurotoxicology 2002; 23 (4–5): 621–33.
68. Morato GS, Lemos T, Takahashi RN. Acute exposure to maneb alters some behavioral functions in the mouse. Neurotoxicol Teratol 1989; 11: 421–425.
69. Takahashi RN, Rogerio R, Zanin M. Maneb enhances MPTP neurotoxicity in mice. Res. Commun. Chem Pathol Pharmacol 1989; 66: 167–170.
70. Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS mangenese intoxication. Neurology 1988; 38: 550–553.
71. Meco G, Bonifati V, Vanacore N, Fabrizio E. Parkinsonism after chronic exposure to the fungicide maneb (manganese ethylene-bis-dithiocarbamate. Scand J Work Environ Health 1994; 20: 301–305.
72. Soleo L, Defazio G, Scarselli R, Zefferino R, Livrea P, Foa V. Toxicity of fungicides containing ethylene-bis-dithiocarbamate in serumless dissociated mesencephalic-striatal primary co-culture. Arch Toxicol 1996; 70: 678–682.
73. Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol 1998; 150: 263–271.
74. Crofton KM, Boncek VM, MacPhail RC. Evidence for monoaminergic involvement in triadimefon-induced hyperactivity. Psychopharmacology 1989; 97: 326–330.
75. Walker QD, Lewis MH, Crofton KM, Mailman RC. Triadimefon, a triazole fungicide, induces stereotyped behavior and alters monoamine metabolism in rats. Toxicol Appl Pharmacol 1990; 102: 474–485.
76. Perkins AN, Eckerman DA, MacPhail RC. Discriminative stimulus properties of triadimefon: Comparisons with methylphenidate. Pharmacol Biochem Behav 1991; 40: 757–761.
77. Walker QD, Mailman RC. Triadimefon and triadimenol: Effects on monoamine uptake and release. Toxicol Appl Pharmacol 1996; 139: 227–233.
78. Ikaiddi ML), Akunne HC, Soliman KFA. Behavioral and neurochemical effects of acute and repeated administration of triadimefon in the male rat. Neurotoxicology 1997; 18: 771–780.
79. Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson's disease? Brain Res 2000; 873(2): 225–34.
80. Tuchsen F, Jensen AA. Agricultural work and the risk of Parkinson's disease in Denmark, 1981–1993. Scand J Work Environ Health 2000; 26 (4): 359–62.
81. Greenamyre JT, Sherer TB, Betarbet R, Panov AV. Complex Parkinson's disease and I. IUBMB Life 2001; 52 (3–5): 135–41.
82. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 2000, 3; 12: 1301–1306.
83. Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol 2003; 179 (1): 9–16.
84. Mandel S, Weinreb O, Youdim MB. Using cDNA microarray to assess Parkinson's disease models and the effects of neuroprotective drugs. Trends Pharmacol Sci 2003; 24 (4): 184–91.
85. Grunblatt E, Mandel S, Maor G, Youdim MB. Gene expression analysis in N-methyl-4-phenyl-l, 2, 3, 6-tetrahydropyridine mice model of Parkinson's disease using cDNA microarray: effect of R-apomorphine. J Neurochem 2001; 78'(1): 1–12.
86. Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 1999; 23(3): 435–47.
87. Tan EK, Tan C, Fook-Chong SM, Lum SY, Chai A, Chung H, Shen H, Zhao Y, Teoh ML, Yih Y, Pavanni R, Chandran VR, Wong MC. Dose-dependent protective effect of coffee, tea, and smoking in Parkinson's disease: a study in ethnic Chinese J Neurol Sci 2003; 216(1): 163–7.
88. Weinreb O, Mandel S, Youdim MB. cDNA gene expression profile homology of antioxidants and their antiapoptotic and proapoptotic activities in human neuroblastoma cells. FASEB J 2003; 17(8): 935–7.
89. Youdim MB. What have we learnt from cDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinson's disease? J Neural Transm Suppl 2003; 65: 73–88.
90. Manyam BV. Paralysis agitans and levodopa in Ayurveda in ancient Indian Medical treatise. Mov Dis 1990; 5 (1): 47–48.
91. Hoenicka J, Vidal L, Morales B, Ampuero I, Jimenez-Jimenez FJ. Berciano J, del Ser T, Jimenez A, Ruiz PG, de Yebenes JG. Molecular findings in familial Parkinson disease in Spain. Arch Neurol 2002; 59 (6): 966–70.
92. Napolitano M, Centonze D, Calce A, Picconi B, Spiezia S, Gulino A, Bernardi G, Calabresi R Experimental Parkinsonism modulates multiple genes involved in the tranduction of dopaminergic signals in the straitum. Neurobiol Dis 2002; 10: 387–395.
93. Mandel S, Grunblatt E, Youdim M. cDNA microarray to study gene expression of dopaminergic neurodegeneration and neuroprotection in MPTP and 6-hydroxydopamine models: implications for idiopathic Parkinson's disease. J Neural Transm Suppl 2000; (60): 117–24.
94. Yoo MS, Chun HS, Son JJ, DeGiorgio LA, Kim DJ, Peng C, Son JH. Oxidative stress regulated genes in nigral dopaminergic neuronal cells: correlation with the known pathology in Parkinson's disease. Brain Res Mol Brain Res 2003; 110 (1): 76–84.
95. Beitner-Johnson D, Seta K, Yuan Y, Kim H, Rust RT, Conrad PW, Kobayashi S, Millhorn DE. Identification of hypoxia-responsive genes in a dopaminergic cell line by subtractive cDNA libraries and microarray analysis. Parkinsonism Relat Disord 2001, 7; 3: 273–281.
96. Hodgson E, Levi PE. Pesticides: an important but underused model for the environmental health sciences. Environ Health Perspect 1996; 104: 97–106.
97. Ecobichon DJ, Joy RM. (1994). Pesticides and neurological diseases. (2nd ed.) Boca Raton: CRC Press.
98. Payami H, Lee N, Zareparsi S, Gonzales McNeal M, Camicioli R, Bird TD, Sexton G, Gancher S, Kaye J, Calhoun D, Swanson PD, Nutt J. Parkinson's disease, CYP2D6 polymorphism, and age. Neurology 2001; 56 (10): 1363–70.
99. Lamba V, Lamba JK, Dilawari JB, Kohli KK. Polymorphism of CYP2D6 in North Indian subjects. Eur J Clin Pharmacol 1998; 54 (9–10): 787–91.
100. Coleman T, Ellis SW, Martin IJ, Lennard MS, Tucker GT. l-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) is N-demethylated by cytochromes P450 2D6, 1A2 and 3A4-implications for Parkinson's disease. J Pharmcol. Exp Ther 1996; 277: 685–690.
101. Tanaka S. Gene diagnosis of Alzhemer's and Parkinson's disease. Rinsho Byori 2002; 50 (10): 965–969.
102. Dragoni S, Bellik L, Frosini M, Sgaragli G, Marini S, Gervasi PG, Valoti M. Deprenyl metabolism by the cytochrome P450 system in monkey (Cercopithecus aethiops) liver microsomes. Xenobiotica 2003; 33 (2): 181–95.
103. Wu RM, Cheng CW, Chen KH, Shan DE, Kuo JW, Ho YF, Chern HD. Genetic polymorphism of the CYP2E1 gene and susceptibility to Parkinson's disease in Taiwanese. J Neural Transm 2002; 109 (11): 1403–14.
104. Abraham BK, Adithan C, Kiran PU, Asad M, Koumaravelou K. Genetic polymorphism of CYP2D6 in Karnataka and Andhra Pradesh population in India. Acta Pharmacol Sin 2000, 21; 6: 494–8.
105. Menegon A, Board PG, Blackburn AC, Mellick GD, Le Coutour DG. Parkinson's disease, pesticides and glutathione transferase polymorphisms. Lancet. 1998; 352: 1344–6.
106. Menegon A, Board PG, Le Coutour DG, Halliday G. The immunohistochemistry of pi class glutathione transferase in the brain in Parkinson's disease. Proc ASCEPT 1998; 5: 158.
107. Pearce RK, Owen A, Daniel S, Jenner R Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in the Parkinson's disease. J Neural Trans 1997; 104: 661–7.
108. Kondo I, Yamamoto M. Genetic polymorphism of paraoxonase I (PON I) and susceptibility to Parkinson's disease. Brain Res 1998; 806: 271–273.
109. Taylor M, Board PG, Mellick GD, Le Coutour DG. Paraoxonase (PON 1 and PON 2) polymorphisms and Parkinson's disease. Proc ASCEPT 1998; 5: 160.
110. Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics 1999; 9 (6): 745–53.
111. Levecque C, Elbaz A, Clavel J, Richard F, Vidal JS, Amouyel R Tzourio C, Alperovitch A, Chartier-Harlin MC. Association between Parkinson's disease and polymorphisms in the nNOS and iNOS genes in a community based case control study. Hum Mol Gen 2003; 12 (1): 79–86.
112. Bialecka M, Drozdzik M, Podraza H. Role of gene polymorphism of catechol-O-methyltransferase (COMT), monoamine oxidase B (MAOB), cytochrome P450 2D6 (CYP2D6) and N-acetyltransferase 2 (NAT2) in pathogenesis of Parkinson's disease. Neurol Neurochir Pol 2002, 36; 1: 113–21.
(c) Annals of Neurosciences.All Rights Reserved