Annals of Neurosciences, Vol 14, No 2 (2007)
Annals of Neurosciences, Volume 14, Issue 2 (April), 2007
SPINAL DORSAL HORN GENE EXPRESSION FOLLOWING LOOSE LIGATION OF THE RAT SCIATIC NERVE
Corresponding Author
Daniel K. Resnick
Department of Neurological Surgery
University of Wisconsin
K4/834 Clinical Science Center
600 Highland Avenue
Madison, WI 53792, USA
Abstract
In this study we examined whether the manifestation and loss of thermal hyperalgesia (TH) was associated with a differential pattern of gene expression in the spinal dorsal horn. In animals exhibiting TH 7 days after loose ligation of the sciatic nerve 8 genes were up-regulated and 9 were down-regulated. In contrast, in animals exhibiting loss of TH 28 days after ligation, only 2 genes were up-regulated and 4 were down-regulated. An interesting pattern of gene expression emerged in comparing the two sciatic ligation groups. Four genes were up-regulated during TH and then down-regulated following its loss. Four other genes exhibited the reverse pattern. These data revealed that in animals exhibiting a loss of TH following peripheral nerve injury the pattern of gene expression was similar to that in control, uninjured animals, and that in both of these groups the pattern was different from that of animals' still exhibiting TH. These data lent further support to the notion that genetic plasticity in the spinal dorsal horn may be an important contributor to peripheral nerve injury-elicited neuropathic pain (NP).
Key words: Neuropathic Pain, Nociception; Peripheral nerve injury, Sciatic nerve; Synaptic plasticity.
Introduction
The processing of nociceptive information in the spinal dorsal horn may change significantly following peripheral nerve injury or inflammation that might lead to the development of persistent pain. We have been using the well-established Bennett and Xie [1] loose ligation of the sciatic nerve model of NP to delineate how selected aspects of sensory information processing in the spinal dorsal horn may be modified by injury. An especially useful feature of this model is the reversibility of the TH that is one of the behavioral signs of NP. This allows for comparisons of dorsal horn sensory processing during the manifestation and after the resolution of the NP behavior. We previously reported that TH was accompanied by activity-dependent long-lasting synaptic plasticity in the superficial spinal dorsal horn, and that the disappearance of the TH coincided with a loss of the long-lasting synaptic plasticity [2]. More recently we reported that there was a similar association between the TH and activation of cyclic AMP response element binding protein (CREB), and that this activation was also reversible because it lasted only as long as the TH [3]. These studies suggested a significant but reversible shift in the manner in which spinal neurons processed sensory information following peripheral nerve injury, and they lent further support to the notion that plasticity in the spinal dorsal horn underlies the eventual development of NP. In the present study we focused on the two behaviorally important time points and examined the gene expression pattern in the spinal dorsal horn of animals subject to loose ligation of their sciatic nerve. Our primary goal was to establish whether more global but differential changes in gene expression coincide with the manifestation and loss of TH. Several recent studies reported global changes in gene expression following peripheral nerve or spinal cord injury [4–13]. However, these studies did not employ the loose ligation model and their focus was not on a comparison between animals exhibiting NP and those in which the pain had resolved.
Materials and Methods
Sciatic ligation and hindpaw withdrawal latency: Male Sprague-Dawley rats (Harlan, ∼300 g) were used. Water and food were provided ad libitum. Experiments were conducted in accordance with guidelines accepted by the International Association for the Study of Pain. The animal protocol was approved by the Animal Care Committee of the School of Veterinary Medicine at the University of Wisconsin-Madison. Twelve animals were anesthetized with isoflurane, and their sciatic nerves loosely ligated as described by Bennett and Xie [1]. Briefly, the sciatic nerve was exposed by blunt dissection, and four ligatures (4.0 chromic gut) spaced about 1 mm apart were tied so that the nerve trunk was just barely constricted when viewed with a dissecting microscope at 40x. Two additional animals were similarly anesthetized and their sciatic nerves were exposed but not ligated. These animals served as sham-operated, surgery controls. The expression and disappearance of TH were assessed with the well-established hind paw withdrawal latency test to a noxious thermal stimulus [14]. Baseline withdrawal latencies were obtained for all animals before they were randomly assigned to a 7 days sham-operated (n=2), 7 days ligated (n=6) or 28 days ligated (n=6) group. At the appropriate times after surgery (7 or 28 days) the hindpaw withdrawal latencies of all animals were obtained again (second behavioral test) before they were anesthetized with isoflurane, euthanized with an intracardiac injection of saturated potassium chloride, and their spinal tissue harvested for further analysis. Six additional animals were not behaviorally tested or subject to surgery. They were anesthetized with isoflurane, euthanized with an intracardiac injection of saturated potassium chloride, and their spinal tissue harvested for further analysis. These animals served as uninjured reference controls. We previously established that baseline, 7 days, and 28 days withdrawal latencies in control, uninjured animals do not change [2,3].
GeneChip and real-time PCR analysis: The methods used for sample preparation, hybridization, data analysis, sensitivity and quantification have been reported [8]. For these experiments, 3 groups of animals were used: control, uninjured (n=3), 7 days ligated (n=3) and 28 days ligated (n=3). The lumbar spinal cords of these animals were exposed by laminectomy around L5, and about 2 cm of the spinal tissue was excised. The dorsal horn was then separated from the ventral horn, pooled (three samples in each of the three groups), and the RNA extracted using procedures described in the Affymetrix GeneChipTM expression analysis manual (Affymetrix Inc., Santa Clara, CA), and reported previously [8, 9, 13]. Real-time PCR was used to detect mRNA levels of selected genes (e.g., C4 complement, MAP2, SNAP-23, B1 and ClC-5) in control, uninjured, (n=3), 7 days sham-operated (n=2), 7 days ligated (n=3) and 28 days ligated (n=3) animals. The lumbar spinal cords of these animals were exposed by laminectomy around L5, and about 2 cm of the spinal tissue was excised. The dorsal horn was then separated from the ventral horn, and further divided into ipsilateral and contralateral quadrants for each animal in each of the four groups before the quadrants were individually processed for the RT-PCR analysis. The amplified transcripts were quantified using the comparative CT method.
Results
The 7 days ligated animals exhibited significantly shortened withdrawal latencies on the second test in the affected leg, 5.5±0.3s (mean ± SEM) vs. 8.0±0.3, F(1,5)=127.5, p<0.001. This is the time of maximal hyperalgesia for the chronic constriction injury model in our hands. In contrast, there were no significant differences between baseline and second test latencies in the 28 days sciatic ligation animals, 8.2 ± 1.0s vs. 8.7±0.9s, F (1,5) =0.6, p=0.5. The disappearance of TH is a normal feature of this NP model although the time at which hyperalgesia disappears varies. In our hands, behavioral signs of TH typically disappear by 21–24 days after sciatic ligation surgery. As previously established, there were no significant differences between baseline and 7 days withdrawal latencies in sham-operated animals (sciatic nerve exposure without ligation), 9.0±0.7s vs. 9.1±1.0s, F(1,2)=0.2, p=0.7. In other words, like control, uninjured animals, the sham-operated animals do not develop TH. Additional statistical analysis confirmed that none of the animals groups differed in their baseline latencies, F (2, 11) =0.2, p=0.8. However, the 7 days sciatic ligation animals significantly differed from both the sham-operated and 28 days ligated animals at the time of the second test, F(2,11)=7.4, p<0.01.
A total of 17 spinal dorsal horn genes showed a change of 2-fold or greater in 7 days ligated animals when compared to control, uninjured animals (Table 1). Eight genes were up-regulated: nicotinic receptor subunit a7, C4 and C3 complement, pre-pro complement C3, microtubule- associated protein 2 (MAP2), synaptosomal-associated protein 23 (SNAP-23), glutamate transporter 1 C-terminal binding protein, and the voltage-gated sodium channel 6a. On the other hand, nine genes were down-regulated: RAC protein kinase g, DOPA decarboxylase, somatostatin receptor (SSTR4), calcium ATPase isoform 2, bradykinin receptor 1 (B1), ClC-5 chloride channel, nicotinic receptor subunit b4, C-fos, and C-kinase type II (b-2). In contrast, the gene expression pattern in the spinal dorsal horn of 28 days animals was very similar to that of control, uninjured animals. Two genes were up-regulated, the ClC-5 chloride channel and Pk248 (a serine-threonine protein kinase), while four genes were down-regulated, synaptojanin, myelin/oligodendrocyte glycoprotein (MOG), vascular cell adhesion molecule (VCAM-1), and sterol-C4-methyl oxidase-like (Table 1). An interesting bidirectional pattern of gene expression emerged when comparing the microarray data across animal groups (Fig. 1). Four genes were up-regulated in animals manifesting TH and then down-regulated in animals in which the TH disappeared (nicotinic receptor subunit a7, C4 complement, MAP2 and SNAP-23). Four other genes exhibited the reverse pattern (RAC protein kinase g, ClC-5, B1 and nicotinic receptor subunit b4).
| GenBank# | Gene name | Function | Fold change |
|---|---|---|---|
| A. 7 days after loose ligation | |||
| Up-regulated | |||
| S53987 | Nicotinic receptor a7 | Neurotransmission | 7.6 |
| U42719 | C4 complement | Immune response | 7.5 |
| AF052596 | SNAP-23 | Membrane fusion | 3.8 |
| X52477 | Pre-pro-complement G3 | Immune response | 3.7 |
| M29866 | C3 complement | Immune response | 2.8 |
| S74265 | MAP2 | Structural protein | 2.7 |
| AF032120 | GLUT1 CBP | Glutamate transporter | 2.0 |
| AA925248 | Volt-gated Na + channel 6a | Membrane excitability | 2.0 |
| Down-regulated | |||
| D49836 | RAC protein kinase g | Transport regulation | 5.9 |
| AI044310 | Dopa decarboxylase | Catecholamine synthesis | 2.5 |
| AJ132230 | Bradykinin 1 receptor | Neurotransmission | 2.2 |
| Z56277 | ClC-5 chloride channel | Neurotransmission | 2.1 |
| U04738 | Somatostatin receptor (SSTR4) | Neurotransmission | 2.1 |
| L05557 | Calcium ATPase isoform 2 | Transporter | 2.1 |
| U42976 | Nicotinic receptor b4 | Neurotransmission | 2.1 |
| X06769 | C-fos | Transcription | 2.0 |
| E01789 | C-kinasetype-II(b-2) | Signal transduction | 2.0 |
| B. 28 days after loose ligation | |||
| Up-regulated | |||
| Z56277 | ClC-5 Chloride channel | Neurotransmission | 2.5 |
| AI069982 | Pk428 (Ser-Thr protein kinase) | Cytoskeleton | 2.0 |
| Down-regulated | |||
| AJ06855 | Synaptojanin | Synaptic vesicle recycling | 2.5 |
| L21995 | Myelin/oligodendrocyte glycoprotein | Myelinogenesis | 2.5 |
| M84488 | Vascular cell adhesion molecule-1 | Inflammation | 2.3 |
| E12625 | Sterol-C4-methyl oxidase-like | Neural repair | 2.0 |
Comparisons were made to control, uninjured animals.
RT-PCR analysis of selected genes confirmed that their expression in either the ipsilateral or contralateral spinal dorsal horn in 7 days sham-operated animals was indistinguishable from that of control, uninjured animals (Table 2). This analysis also confirmed that the genes coding for C4 complement, MAP2 and SNAP-23 proteins were up-regulated in the ipsilateral dorsal horn of 7 days sciatic ligation animals when compared to the same side in 7 days sham-operated animals. On the other hand, the gene coding for bradykinin B1 receptors was down-regulated in these animals exhibiting TH. Similarly, RT-PCR analysis confirmed the up-regulation of the ClC-5 gene in the ipsilateral dorsal horn of 28 days sciatic ligation animals that recovered from the TH. The expression of any of these genes on the contralateral side of the dorsal horn was unchanged (Table 2).
| Sham | 7-days ligated | 28-days | |||||
|---|---|---|---|---|---|---|---|
| ligated GenBank# | Gene name | IL | CL | IL | CL | IL | CL |
| U42719 | C4 complement | -1.4 | 1.2 | 14.6 | 1.1 | ||
| AF052596 | SNAP-23 | -1.3 | 1.5 | 2.1 | 0.8 | ||
| S74265 | MAP2 | -1.8 | 1.7 | 2.0 | 0.9 | ||
| AJ132230 | Bradykinin B1 | 1.6 | 1.0 | -2.7 | 0.3 | ||
| Z56277 | ClC-5 Cl channel | -1.1 | 1.2 | 2.1 | -1.9 | ||
Discussion
Our results revealed a differential pattern of gene expression in the spinal dorsal horn following peripheral nerve injury, between animals manifesting TH and animals in which this behavioral sign of NP had resolved. In the latter animals, the gene expression pattern was very similar to that of control, uninjured animals. These data are in agreement with studies reporting global changes in gene expression in other animal models of either peripheral nerve or spinal cord injury [4–13,15]. In all of these models, the genes whose expression is either up- or down-regulated play important roles in inflammation, neurotransmission or signal transduction. Similarly, their protein products have been postulated to play significant roles in the development and maintenance of injury-elicited chronic pain [16,17]. We find it intriguing that the gene expression pattern in animals that recovered from the TH was very similar to that in control, uninjured, or in sham-operated animals. This implies that compensatory changes in gene expression in the spinal dorsal horn may contribute to the recovery from NP. Our finding that several genes were regulated in a bidirectional pattern during and after the manifestation of TH further supports this notion.
C4 complement protein plays a role in the immune response [18], MAP2 is a cytoskeletal phosphoprotein that regulates the dynamic assembly of microtubules [19], and SNAP-23 is involved with synaptic vesicle release [20]. Our data suggest an association of all three proteins with NP. In contrast, recovery from TH was accompanied by up-regulation of the gene for RAC protein kinase g (a.k.a. AKT-3 or protein kinase B g). This kinase regulates the function of many cellular proteins in metabolism, apoptosis, and proliferation but its role in the spinal dorsal horn remains unknown[21] . On the other hand, nicotinic receptor agonists appear to be a new class of effective analgesics and specific receptor subunits may thus be associated with NP while others with its loss[22].
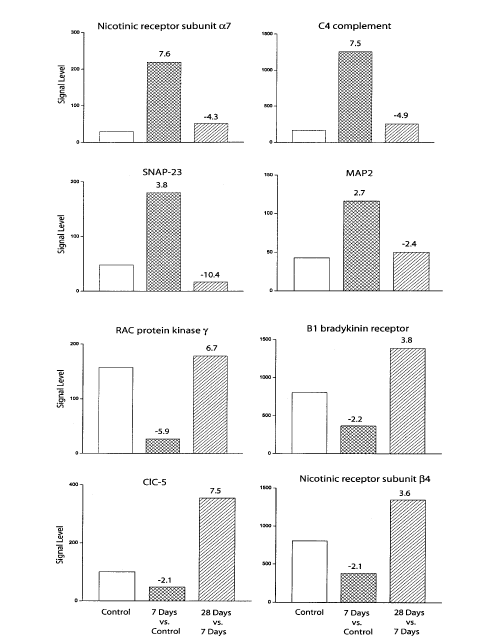
Figure 1. The manifestation and loss of TH produced a bidirectional pattern of gene expression in the spinal dorsal horn. Gene expression levels were compared first between the 7 days ligated and the control group, and then between the 7 and 28 days ligated groups. Note that four genes were up-regulated in animals manifesting NP behavior and then down-regulated following the loss of this behavior. Four other genes exhibited the reverse pattern. Numbers above each column indicate fold changes.
It is further intriguing that the gene coding for ClC-5, a member of the voltage-gated chloride channel family, was one of only two genes up-regulated in 28 days animals exhibiting a loss of TH (Table 2). This up-regulation was especially prominent when compared to the down-regulation of the ClC-5 gene in animals manifesting NP (Fig. 2). Nothing is presently known about the role of these channels in the processing of sensory information in the spinal dorsal horn. In general, chloride channels mediate GABA inhibitory activity, and a recent study reports a critical role for another member of this family in altering GABA-inhibitory neurotransmission in the hippocampus of ClC-3 deficient knockout mice [23]. It is probable that ClC-5, or the other members of this family, play a similar critical role in promoting GABA inhibition in the spinal dorsal horn.
Inhibitory activity of neurons is considered critical in setting the conditions for activity-dependent plasticity in the hippocampus, and the greater the degree of this inhibition the less likely it is for long-lasting excitability to develop [24]. In the absence of effective inhibition in the spinal dorsal horn the injury-elicited repetitive stimulation of primary afferents may be reflected in enhanced sensitivity to both innocuous and noxious peripheral stimuli, i.e., allodynia and hyperalgesia. Consequently, restoration of inhibition in the spinal dorsal horn may be a critical step in the recovery from TH, and by extension, in the recovery of normal pain sensibility.
More difficult to explain is a similar pattern of change in the expression of the gene coding for bradykinin 1 (B1) receptors (Fig. 2) because their activation appears to mediate inflammatory mechanical hyperalgesia [25]. However, given that different molecular mechanisms may underlie different aspects of chronic pain states [16] Our data imply that the TH elicited by loose ligation of the sciatic nerve is not dependent on activation of B1 receptors. In fact, their up-regulation in recovered animals may provide yet another indication of the recovery of normal pain sensibility in these animals, and in the similarity in the processing of nociceptive information between these animals and their control, uninjured counterparts.
In summary, our results revealed a differential pattern of gene expression in the spinal dorsal horn following loose ligation of the sciatic nerve between animals manifesting TH and animals in which this behavioral sign of NP had resolved. These data lent further support to the notion that genetic plasticity in the spinal dorsal horn may be an important contributor to the NP elicited by peripheral nerve injury.
Acknowledgements
Supported in part by NIH grants NS 044173 (RV) and NS 034870 (VM).
References
1. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87–107.
2. Draganic R Miletic G, Miletic V. Changes in post-tetanic potentiation of A-fiber dorsal horn field potentials parallel the development and disappearance of NP after sciatic nerve ligation in rats. Neurosci Lett 2001; 301: 127–30.
3. Miletic G, Pankratz MT, Miletic V: Increases in the phosphorylation of cyclic AMP response element binding protein (CREB) and decreases in the content of calcineurin accompany NP following chronic constriction injury in rats. Pain 2002; 99: 493–500.
4. Costigan M, Befort K, Karchewski L et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 2002; 3: 16.
5. Fan M, Mi R, Yew DT, Chan WY: Analysis of gene expression following sciatic nerve crush and spinal cord hemisection in the mouse by microarray expression profiling. Ceil Mol Neurobiol 2001; 21: 497–508.
6. Ko J, Na DS, Lee YH et al. cDNA microarray analysis of the differential gene expression in the NP and electroacupuncture treatment models. J Biochem Mol Biol 2002; 35: 420–27.
7. Rabert D, Xiao Y, Yiangou Y et al. Plasticity of gene expression in injured human dorsal root ganglia revealed by GeneChip oligonucleotide microarrays. J Clin Neurosci 2004; 11: 289–99.
8. Resnick DK, Schmitt C, Miranpuri GS et al.: Molecular evidence of repair and plasticity following spinal cord injury. Neuroreport 2004; 15: 837–39.
9. Song G, Cechvala C, Resnick DK et al. : GeneChip analysis after acute spinal cord injury in rat. J Neurochem 2004; 79: 804–15.
10. Sun H, Xu J, Della Penna KB et al. Dorsal horn-enriched genes identified by DNA microarray, in situ hybridization and immunohistochemistry. BMC Neurosci 2002; 3: 11.
11. Tachibana T, Noguchi K, Ruda MA: Analysis of gene expression following spinal cord injury in rat using complementary DNA microarray. Neurosci Lett 2002; 327: 133–37.
12. Valder CR, Liu JJ, Song YH, Luo ZD: Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem 2003; 87: 560–73.
13. Wang H, Sun H, Della Penna K et al. Chronic NP is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience 2002; 114: 529–46.
14. Hargreaves K, Dubner R, Brown F et al.: A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88.
15. Resnick DK, Graham SH, Dixon CE, Marion DW: Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma 1998; 15: 1005–13.
16. Ji RR, Kohno T, Moore KA, Woolf CJ: Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 2003; 26: 696–705.
17. Siddall PJ, Loeser JD. Pain following spinal cord injury: Spinal Cord 2001; 39: 63–73,
18. Blom AM, Villoutreix BO, Dahlback B. Complement inhibitor C4b-binding protein-friend or foe in the innate immune system? Mol Immunol 2004; 40: 1333–46.
19. Buddle M, Eberhardt E, Ciminello LH et al. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res 2003; 978: 38–50.
20. Dietrich LE, Boeddinghaus C, LaGrassa TJ, Ungermann C. Control of eukaryotic membrane fusion by N-terminal domains of SNARE proteins. Biochim Biophys Acta 2003; 1641: 11–119.
21. Nicholson KM, Anderson NG: The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 2002; 14: 381–95.
22. Decker MW, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: a potential new class of analgesics. Curr Top Med Chem 2004; 4: 369–84,.
23. Dickerson LW, Bonthius DJ, Schutte BC et al. Altered GABAergic function accompanies hippocampal degeneration in mice lacking ClC-3 voltage-gated chloride channels. Brain Res 2002; 958: 227–50.
24. Paulsen O, Moser EI: A model of hippocampal memory encoding and retrieval – GABAergic control of synaptic plasticity. Trends Neurosci 1998; 21: 273–78.
25. Fox A, Wotherspoon G, McNair K et al. Regulation and function of spinal and peripheral neuronal B1 bradykinin receptors in inflammatory mechanical hyperalgesia. Pain 2003; 104: 683–91.
(c) Annals of Neurosciences.All Rights Reserved