Annals of Neurosciences, Vol 14, No 3 (2007)
Annals of Neurosciences, Volume 14, Issue 3 (July), 2007
HISTOENZYMOLOGICAL DISTRIBUTION OF ACETYLCHOLINESTERASE IN THE CEREBRAL HEMISPHERES OF INDIAN WALL LIZARD, HEMIDACTYLUS FLAVIVIRIDIS
Corresponding author
Prof. U C Srivastava
Professor, Department of Zoology University of Allahabad, Allahabad-211 002 INDIA
E-mail:
Abstract
The present investigation deals with the histoenzymological distribution of acetylcholinesterase (AChE) in the cerebral hemispheres of Hemidactylus flaviviridis by employing a modified histochemical technique to visualize AChE containing neurons described by J.C. Hedreen et al. (1985). In AChE preparations large number of nuclei exhibit positive reaction varying from moderate to intense, while septal nuclei showed mild activity. Dorsal ventricular ridge (DVR) presented hybrid nature of intensity. In cortical regions, dorsomedial cortex demonstrated intense reaction at all levels. The lateral forebrain bundle was negative while medial forebrain bundle was positive for the same enzyme. Tractus olfactorius lateralis presented staining of variable nature at all levels.
The data obtained for various sites have been discussed from the functional point of view in the light of recent cytoarchitectonic and connectional studies made by neuroscientists in reptiles.
Key words: Acetylcholinesterase, Neuron, Brain, Lizard, Synapse.
Introduction
Acetylcholine (ACh) is the major neurotransmitter of the Cholinergic system. ACh is secreted from the presynaptic nerve terminal and binds to acetylcholine receptors which are clustered in the post synaptic membrane. After release ACh is rapidly removed from the synaptic cleft by acetylcholinesterase (AChE), which belongs to the family of type B Carboxylesterase and cleaves ACh in to Choline and acetate. (1).
In recent years many non cholinergic functions of AChE have been elucidated besides its main cholinergic role (2,3,4). These wide functions of AChE provide adequate base to functionally correlate its variable distribution in the different nuclei of the brain. The distribution of Cholinesterases in mammalian brains (5–10) and avian brains (11,12,13) and their possible roles have been studied by earlier workers. The study of distribution pattern of AChE in reptiles is confined and based on old topographical neuroanatomy (14,15,16).
The present investigation was carried out with an aim to histoenzymologically map the cerebral hemispheres of Indian wall lizard for AChE preparations, which seems to have escaped the attention of workers, in the light of recent topological and connectional studies (17,18,19). In addition to this the second objective of the present investigation was to compare the present findings with that of other reptiles, worked out by earlier scientists and to establish a correlation between the functional significance of the enzyme related with mammals and its wide distribution in the different centres of cerebral hemispheres in the presently studied animal.
Materials and Methods
Ten adult wall lizards were sacrificed in the present investigation. The experiments were conducted in confirmity with the ethical guidelines of University of Allahabad, Allahabad. Lizards were decapitated without anesthesia and the brains were immediately taken out. Tissue was fixed in 0.5% paraformaldehyde and 1.5% gluteraldehyde in 0.1 M phosphate buffer (pH 7.2) for six hours at 4°C. After six hrs. the tissue was given 2–3 changes in 15% sucrose solution in 0.1 M phosphate buffer and stored in the same solution for 2–3 days. 40 mm thick frozen, sections were cut by AO Histostat at -18°C and processed for AChE using a modified histochemical procedure to visualize AChE containing neurons (20). Suitable controls were also made simultaneously.
Results
The identification of various nuclei and brain regions is based on the earlier study of Northcut (1967) and recent study of Aboitiz et al. (1995,1999,2002). For a coordinated study, the sections were taken at the levels of anterior, middle and posterior region.
Cortical Centres
The roof of the telencephalon is occupied by cerebral cortex. The cerebral cortex of the presently studied animal has been differentiated in to medial cortex (MCx), Dorsomedial Cortex (DMCx), Dorsal Cortex (DCx) and lateral Cortex (LCx) (Figs. 1–5). Each Cortical area forms a rostrocaudally oriented strip on the hemisphere. MCx and DMCx are present as distinct areas through out most of the cortex while DCx is present only in the intermediate sector, LCx extends laterally, the full langth of the hemisphere. Each cortical area consists of three distinct layers (Figs. 1–5). The number and nature of neurons and the thickness of the layers varies from one cortical centre to other. In the present observation the dorsal Cortex (DCx) has been divided in to medial (DCm) and lateral parts (DCl). The AChE activity in the different layers of above described cortical regions was as follows: (Table 1)
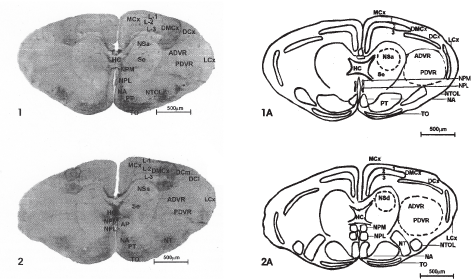
Fig 1–2: Photomicrographs of 40mm thick cryocut transverse sections passing from rostral region of cerebral hemispheres 4 X.
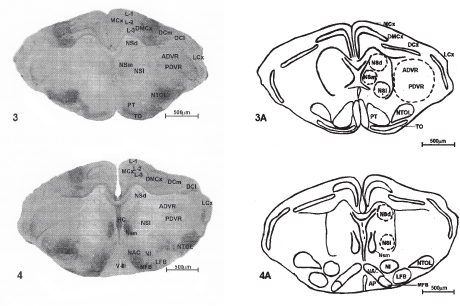
Fig 3–4 Photomicrographs of 40mm thick cryocut transverse sections passing through the middle level of cerebral hemispheres. 4 X.
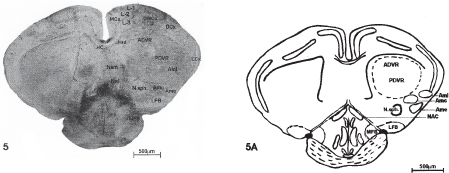
Fig 5- Photomicrographs of 40mm thick cryocut transverse section passing through caudal level of cerebral hemispheres. 4 X.
Fig 1A-5A: Schematic representation, showing the configuration of various nuclei and fibre tracts from restral to caudal levels of the cerebral hemispheres of Hemidactylus flauiviridis.
Abbreviations used in figures: MCx- Medial Cortex; DMCx - Dorsomedial Cortex; DCx- Dorsal Cortex; DCm- Medial part of dorsal Cortex; Del- Lateral part of dorsal Cortex; LCx- Lateral Cortex; L 1-Layer I, L 2 - Layer II, L 3- Layer III; ADVR- Anterior dorsal Ventricular ridge; PDVR- Posterior dorsal Ventricular ridge; Se- Septum; NSa- Nucleus septalis anterior; HC- Hippocampal Commissure; NSl- Nucleus septalis lateralis; NSm- Nucleus septalis medialis; NSd- Nucleus septalis dorsalis; TO- Tuberculum olfactorium; AP- Area prolifactoria; NPM- Nucleus parol factorius medialis; NPL- Nucleus parolfactorius lateralis; N1- Nucleus interstitialis; NTOL- Nucleus tractus olfactorius lateralis; NA-Nucleus accumbence; PT- Paleostriatum; NAC- Nucleus Commissural anterioris; LFB- Lateral forebrain bundle; MFB-Medial fore brain bundle; N.sph- Nucleus sphericus; Ami- nucleus lateralis amygdali; AMC- Nucleus Centrale amygdali; Ame- Nucleus external amygdali; V-III - Third ventricle.
Layer I
In MCx region layer I showed varying intensity for AChE. In the lower region it was highly intense while in the upper region, towards DMCx, it was comparatively less intense (Fig. 1). In the DMCx region this layer showed high intensity, greater in comparison to MCx and DCx. Its intensity gradually increased towards DCx region (Figs. 1–2). In the DCx region layer I showed intense activity as compared to MCx but less than DMCx region (Figs. 1–2). In DMCx region, all the three layers showed very intense reaction for AChE and it was difficult to differentiate between these layers.
The medial part of the dorsal Cortex (DCm) was more intense in comparison to its lateral counterpart (DCl). (Fig. 2). In LCx region Layer I showed less intense reaction in comparison to same layer in dorsal, dorsomedial and medial Cortex (Fig. 2).
| S.No. | Layers | Abbreviation | AChE Activity | Fig. No. |
|---|---|---|---|---|
| 1. | Layer I | L-I | 1,2 | |
| (a) Medial Cortex | MCx | + + | ||
| (b) Dorsomedial Cortex | DMCx DCx | + + + | ||
| (c) Dorsal Cortex | DCx | |||
| (i) Pars medialis part of DCx | DCm | + + + | ||
| (ii) Pars lateralis part of Dorsal cortex | DCl | + + | ||
| (d) Lateral cortex | LCx | + − | ||
| 2. | Layer II | L-2 | 1,2 | |
| (a) Medial Cortex | MCx | -- | ||
| (b) Dorsomedial Cortex | DMCx | + + + | ||
| (c) Dorsal Cortex | DCx | |||
| (i) Pars medialis part of DCx | DCm | + + | ||
| (ii) Pars lateralis part of Dorsal cortex | DCl | + − | ||
| (d) Lateral cortex | LCx | + − | ||
| 3. | Layer III | L-3 | 2–5 | |
| (a) Medial Cortex | MCx | + + | ||
| (b) Dorsomedial Cortex | DMCx | + + + | ||
| (c) Dorsal Cortex | DCx | |||
| (i) Pars medialis part of DCx | DCm | + + + | ||
| (ii) Pars lateralis part of Dorsal cortex | DCl | + + | ||
| (d) Lateral cortex | LCx |
Layer II
Layer II showed total negativity in the MCx region (Figs. 1, 2) while in the DMCx region it showed very intense but diffused reaction along with layer I and layer III (Figs. 1, 2) In the DCx region this layer showed intense activity but in the LCx region it showed mild reaction in both dorsal and ventral regions. (Fig. 2)
Layer III
Layer III showed very intense reaction as compared to layer I in the MCx region as well as in the DCx region (Figs. 1,2). In the DMCx region it showed uniform intense activity along with layer I and layer II (Figs. 1,2). In the LCx, layer III was intense. (Figs. 2, 3) The intensity of cortical layers gradually increased from rostral to middle regions but interestingly it decreased in caudal regions. (Fig. 5) and showed uniform mild reaction in medial, dorsal and lateral cortical regions but it still showed intense reaction in dorsomedial cortex region, which was less intense than dorsomedial cortices of rostral and middle regions.
However gross observations under the higher magnification (not visible in photograph) clearly revealed the AChE reaction in perikaryen, dendrites as well as neuropil. But it was difficult to delineate the three Cortical layers in the DMCx region because all three layers demonstrated high intense but uniform activity for AChE.
Dorsal Ventricular ridge (DVR)
Below the layers of Cortex, a region called dorsal ventricular ridge (DVR) is present which bulges in to lateral ventricle, located above basal ganglia. The DVR has been divided in to anterior dorsal ventricular ridge (ADVR) and posterior dorsal ventricular ridge (PDVR) (Fig. 1–5). In the present study ADVR has been distinguished into mediodorsal, dorsalateral, medioventral and ventrolateral parts while PDVR has been distinguished in to medial and lateral parts. The intensity of AChE reaction varied in these regions as follows: (Table 2)
| S.No. | ADVR | AChE Activity | Fig. No. |
|---|---|---|---|
| 1. | Anterior section | 1,2 | |
| (a) Mediodorsal | + + | ||
| (b) Dorsolateral | + + + | ||
| (c) Medioventral | + − | ||
| (d) Mediolateral | + + | ||
| 2. | Middle section | 3,4 | |
| (a) Mediodorsal | + + | ||
| (b) Dorsomlaterial | + + + | ||
| (c) Mediovental | + − | ||
| (d) Mediolateral | + + | ||
| 3. | Posterior section | 5 | |
| (a) Mediodorsal | + + | ||
| (b) Dorsolateral | + + + | ||
| (c) Medioventral | + + | ||
| (d) Mediolateral | + + + | ||
| PDVR | |||
| 4. | Anterior section | 1,2 | |
| (a) Medial | + + | ||
| (b) Lateral | + + + | ||
| 5. | Middle section | 3,4 | |
| (a) Medial | + | ||
| (b) Lateral | + + + | ||
| 6. | Posterior section | 5 | |
| (a) Medial | + − | ||
| (b) Lateral | + + |
ADVR
AChE activity in entire ADVR increased from rostral to caudal sections. In the anterior sections the dorsolateral and mediolateral parts were highly intense (Fig. 2) while mediodorsal and medioventral regions were comparatively less intense (Fig. 2) the same kind of activity was also seen in middle section. (Fig. 3), but interestingly the entire ADVR region was intense in caudal section (Fig. 5). In all the sections, the ADVR adjacent to PDVR was more intense in comparison to other regions.
PDVR
In anterior sections the lateral part of PDVR was comparatively more intense than the medial part while in the middle sections, the lateral part was highly intense but the medial part showed mild intensity. (Figs. 3,4). In the posterior sections, both medial and lateral parts were mild intense (Fig. 5).
SEPTUM
The medial wall of the hemisphere is mainly occupied by the septal area. In wall lizard four subdivisions have been recognized. A nucleus septalis anterior consisting of loosely arranged cells is present in the rostral half of the hemisphere (Fig. 1). At mid hemispheric level this nucleus is replaced by the posterior septal nucleus, which can be sub divided into lateral and medial parts (Figs; 3,4). The nucleus septalis lateralis is the largest of the two subdivisions. It can be traced caudally to the level of the posterior hippocampal commissure (Figs. 4 5). The nucleus septalis medialis is situated rostrally close to the midline (Fig. 3), but caudally it lies adjacent to the lateral septal nucleus (Fig. 5).
In addition to these three nuclei nucleus septalis dorsalis has also been recognized, which extends throughout the caudal half of the hemisphere and is separated from the other septal nuclei by fibres of the anterior commissure (Fig. 4).
In the rostral region of the cerebral hemisphere nucleus septalis anterior showed negativity for AChE preparations (Fig. 1) while hippocampal commissure (HC) exhibited very intense reaction. In the middle region as well as in the caudal region, nucleus septalis lateralis revealed mild activity for AChE (Figs. 4, 5). Nucleus septalis dorsalis showed negative reaction in all regions (Figs. 3,4). Nucleus septalis medialis demonstrated mild activity in middle regions while it revealed intense reaction in caudal regions (Figs. 4, 5). Hippocampal commissure showed intense reaction in middle regions (Fig. 4) while it revealed mild activity for AChE preparation in caudal region (Fig. 5) (Table 3).
| S.No. | Nuclei/Commissure | Abbreviation | AChE Activity | Fig. No. |
|---|---|---|---|---|
| 1. | Anterior region | 1,2 | ||
| Nucleus septalis anterior | Nsa | − − | ||
| Hippocampal Commissure | HC | + + + | ||
| 2. | Middle region | 3,4 | ||
| Nucleus septalis lateralis | Nsl | + − | ||
| Nucleus septalis medialis | Nsm | + − | ||
| Nucleus septalis dorsalis | Nsd | − − | ||
| Hippocampal Commissure | HC | + + | ||
| 3. | Caudal region | 5 | ||
| Nucleus septalis lateralis | Nsl | + − | ||
| Nucleus septalis medialis | Nsm | + + | ||
| Nucleus septalis dorsalis | Nsd | − − | ||
| Hippocampal Commissure | HC | + − |
Amygdala
The most conspicuous area in the amygdaloid complex is the nucleus sphericus (N. sph.) whose cells are arranged in a cortex like fashion (Fig. 5).
Apart from nucleus sphericus three other amygdaloid nuclei have been recognized viz; nucleus externus amygdali (Ame), nucleus medialis amygdali (Amm) and nucleus lateralis amygdali (Aml) (Fig.5).
In histoenzymological preparations N. sph demonstrated mild reaction for AChE preparations, almost in all levels (Fig. 5). Nucleus amygdali lateralis revealed intense activity (Fig. 5). While nucleus external amygdali (Ame) and nucleus centralis amygdali (Amc) showed mild activity (Fig.) for AChE preparations (Table 4).
| S.No. | Name of nuclei | Abbreviation | AChE activity | Fig. No. |
|---|---|---|---|---|
| 1 | Nucleus sphericus | N.sph | + − | 5 |
| 2 | Nucleus lateralis amygdali | Aml | + − | 5 |
| 3 | Nucleus centrale amygdali | Amc | + + | 5 |
| 4 | Nucleus external amygdali | Ame | + − | 5 |
Striatum Centres
It includes tuberculum olfactorium (TO), situated ventral to the nucleus of the lateral olfactory tract (NTOL) and anterior to paleostriatum (PT); Area prolifactoria, located lateral to the nucleus hippocampi and dorsal to nucleus accumbens (NA). Area prolifactoria (AP) is divided into two nuclei namely nucleus parolfactorium lateralis (NPL) and nucleus parolfactorium medialis (Fig. 2). Nucleus commissurae anterioriris (NAC) lies among the fibres of anterior commissurae and nucleus septalis medialis (Nsm) (Fig. 5); nucleus interstitialis (Nl) is bordered dorsally by DVR and ventrally by lateral forebrain bundle (LFB);nucleus preopticus (NP) is situated on the lateral side of third ventricle (V-III) and is bordered dorsally by NAC (Fig. 4).
In the topoenzymological study, TO revealed intense activity while NPM and NPL showed slightly less activity (Fig. 1). In the rostral section NPM and NPL showed less activity which gradually increased in the caudal sections while TO showed uniform activity in all the sections. N1 showed moderate activity. Paleostriatum NA and NTOL showed very intense activity (Figs. 2,3) (Table 5).
| S.No. | Striatum centre/Fibre Bundles | Abbreviation | AChE Activity | Fig. No. |
|---|---|---|---|---|
| 1. | Tuberculum olfactorium | TO | ++ + | 1,2 |
| 2. | Nucleus parolfactorius mediate | NPM | ++ + | 1,2 |
| 3. | Nucleus parolfactorium lateralis | NPL | + + + | 1,2 |
| 4. | Nucleus Commissural anterioris | NAC | + + | 4,5 |
| 5. | Nucleus interstitialis | NI | ++ | 4 |
| 6. | Nucleus tractus olfactorius lateralis | NTOL | ++ | 3,4 |
| 7. | Nucleus accumbense | NA | ++ + | 2 |
| 8. | Paleostriatum | PT | + + + | 2,3 |
| 9. | Lateral forebrain bundle | LFB | - | 4,5 |
| 10. | Medial forebrain bundle | MFB | ++ + | 4,5 |
Notation
+ + + = Very intense, + + = Intense, +-= Mild, - - = Negative
Fibre Bundles
Two types of bundles namely lateral forebrain bundle (LFB) and median forebrain bundle (MFB) are located in the basal region of forebrain. They are well distinguished in middle sections (Fig. 4). LFB revealed negative activity for AChE in rostral sections but few cells showed positive reaction. MFB revealed very intense activity in all the sections (Figs. 4, 5).
NTOL, in the form of compact group was present at all the levels. This tract is surrounded at the middle and posterior levels by N. Sph. at a distance. Its intensity increased from rostral to caudal section (Table 5).
Discussion
In the present investigation the high intense activity was seen in ten nuclei (TO, NTOL, PT, NA, NT, Nsm, NPM, NPL, NI, NAC) while ADVR and HC demonstrated intense activity in rostral and middle regions and in caudal regions these showed decreased activity for AChE. Contrary to ADVR, PDVR showed moderate activity almost in all regions but its lateral portion adjacent to LCx showed intense reaction. Cortical centres demonstrated moderate reaction except dorsomedial Cortex (DMCx) which showed intense activity in all three cell layers.
The ten intensely stained nuclei in the presently studied animal are motor as observed by Goldby (1937) (21). In the category of the moderate nuclei, majority of the nuclei are also motor. In the cortical area, most of the nuclei are also motor except DMCx which is sensory as mentioned by Sethi & Tewari (1977) in Uromastix (14). DVR, which from the view of staining, presented a hybrid nature, is also motor, that is in consistence while comparing the present results with that of Sethi & Tewari (1977) in Uromastix where hyperstriatum (in our terminology DVR), demonstrating the variable intensity was motor in nature. In our results NPM & NPL revealed intense activity while these were moderate in the findings of Sethi & Tewari (1977) in Uromastix. However, it is apparent from the present study that most of the motor nuclei are equipped with AChE, either with a high or moderate degree of activity.
Having considered all these findings, it is worth analyzing the various possible causes for differential staining. In present investigations, most of nuclear groups and regions in the cerebral hemisphere were intensely stained for AChE. These might be considered as cholinergic in the light of earlier findings (22) and the low activity of the AChE reflects the non cholinergic nature of neurons. The cholinoceptive cells may often have AChE on their cell membrane even they are non-cholinergic (23). "Acetylcholine splitting enzyme is present in variable concentrations in all types of conducting tissue, nerve and muscle, vertebrate and invertebrate, central and peripheral tissues, motor and sensory fibres, sympathetic and parasympathetic, cholinergic and noncholinergic fibres (24). Therefore it may be suggested that highly intense nuclei reflect the presence of cholinergic neurons while moderate or mild stained nuclei may be noncholinergic in the present study. Further the author remarks that at the regions where cell bodies and junctions are located, concentrations are higher and the myelinated nerve fibres in general have lower concentrations than the unmyelinated and white matter contains much less enzyme than the gray matter. The attachment of AChE to the membrane surface, has been supported by differential centrifugation studies (25,26) and electron microscopic studies (27). In addition to nerve cells, glial cells are also supposed to contain little AChE activity (28).
In our findings ten nuclear groups (TO, NTOL, PT, NA, NT, Nsm, NPM, SPL, NI, NAC) in the cerebral hemisphere showed high intensity it seems that a large number of synapse might be present in the neurons of these nuclei, playing active role in synaptic nerve transmission more over the concentration of unmyelinated nerve fibres also seems to be high in intensely stained nuclei and most of them are concentrated in the grey matter.
Interestingly commissures and tracts demonstrate negativity for AChE in H. flauiviridis showing non cholinergic nature of neurons. These findings are in the line of the earlier findings (14). It is therefore suggested in our study that positive staining in the nuclei at the gross level may have been contributed by neurons and glial cells, the nerve fibres and tracts having no part in the overall picture of the histoenzymological maps.
In isolated motoneurons, AChE activity is about ten times greater in the cytoplasm, dendrites and axons than in the nucleoplasm and no activity is present in the nucleolus (29). Thus the main contribution in the positively stained nuclei for AChE preparations is due to cytoplasm of neurons, axon and dendrites excluding nucleolus.
The presence of a dense background staining of AChE is often suggested to be an accurate enough marker for the simultaneous presence of AChE and ChAT (enzymes, catalysing the synthesis of AChE) and serves as a good indicator, although indirect of both cholinergic and cholinoceptive brain regions (30). But there are also indications of non parallel distribution when simultaneous assays of ChAT and AChE have been conducted (31) which indicate that only a co-occurrence of AChE and ChAT denote cholinergic and cholinoceptive nature of neurons, while AChE staining alone denotes cholinoceptive neurons. AChE presence in the perikarya and neuropil is probably suggestive of both cholinergic and cholinoceptive activity. In our study to identify the accurate nature of neurons in various nuclei, the simultanious assay of AChE and ChAT is required and the highly stained nuclei may be cholinergic or cholinoceptive which needs further investigation by immunocytochemical method.
Other observations (2, 3) have shown that AChE hydrolyses substance R met- and leu-enkephalin and could degrade other neuropeptides as well. This could explain the very widespread staining observed in different nuclear areas of the cerebral hemispheres in the presently studied animal. However, distributions of AChE, ChAT and various acetylcholine receptor sites have served as good indicator of cholinergic system in vertebrates. On the basis of these observations, many direct homologies can be established among various cholinergic regions, fibre tracts and nuclei of the vertebrate brains, making it a suitable technique for comparative analysis of functional neuroanatomy.
Thus the essence of the discussion is that, in the present map, the stained nuclei as a whole reflect the configuration of the neurons exhibiting AChE activity in their perikarya, on their plasma membrane and the associated synapses. And in totality, such areas are helping in the transmission of nerve impulses and playing significant roles in physiological and metabolic processes.
Acknowledgement
This work is supported by the CSIR fellowship F. No. 2–56/2002 (I) EU. II. Authors acknowledge, Head, Department of Zoology, University of Allahabad, Allahabad for providing essential facilities for the present investigation.
References:
1. Soreq H, Seidman S. Acetylcholinesterase: New role for an old actor. Nat Rev Neurosci. 2001; 2 : 294–302.
2. Chub IW, Ranieri E, Hodgson AJ, White GH. The hydrolysis of Leu and Met-enkephalin by acetylcholinesterase. Neurosci Lett 1982; 8 : 539
3. Chub IW, Hodgson AJ, White CH. Acetylcholinesterase hydrolyses substance P. Neuroscience. 1980; 5: 2065–72.
4. Downes GB, Grant M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev Biol 2004; 270: 232–45.
5. Ishii Y. The histochemical studies of Cholinesterase in the central nervous system: Normal distribution in rodents (in Japanese). Arch Histol Jap 1975; 12: 587–611.
6. Shute CCD, Lewis PR. Cholinesterase containing system of the brain of rat. Nature. 1963; 199: 1160–64.
7. Giris M. Distribution of Cholinesterase in the basal rhinencephalic structures of the Coypu (Mycastor ccypus). J Comp Neurol 1967; 129: 85–95.
8. Ishii T, Friede RL. A comparative histochemical mapping of the distribution of acetylcholinesterase and nicotinamide adenine-di nucleotide-diaphorase activities in the human brain. Inter Rev Neurobiol 1967; 10: 231–75.
9. Manocha SL, Shantha TR, Macaca M. Enzyme histochemistry of the nervous system. New York Acadmic press 1970; 115.
10. Bhatt DK, Tewari HB. Histochemical mapping of acetylcholesterase and butyrycholinesterase in the medulla oblongata and pons of squirrel (Funambulus palmerum). J Neurosci 1978; 3: 419–39.
11. Whittaker VD. The specificity of pigeon brain acetylcholinesterase. Biochem. J 1953; 54: 660–64.
12. Cavanagh JB, Holland P. Cholinesterase in the chicken nervous system. Nature. 1961; 190: 735–36.
13. Sadananda M. Acetylcholinesterase in central vocal control nuclei of the zebra finch (Talniopygia guttata). J Biosci 2004; 292: 189–200.
14. Sethi JS, Tewari HB. Histochemical mapping of Cholinesterases in the cerebral hemispheres of Uromastix hardwickii. Cell and Molec Biology. 1977; 22: 263–75.
15. Sethi JS, Tewari HB. Histoenzymological mapping of acetylcholinesterase and butyrylcholinesterase in the diencephalon and mesencephalon of Urmastix hardwickii. J Himforsch 1976; 17: 335–49.
16. Tewari HB, Sethi JS. Topographical distribution of acetylcholinesterase and butyrylcholinesterase in the diencephalon and mesencephalon of the garden lizard (Calotes versicolor). J Neurosci Res 1977; 21–35.
17. Aboitiz F. Homology in the evolution of the cerebral hemispheres: the case of reptilian dorsal ventricular ridege and its possible correspondence with mammalian neocortex. J Himforsch 1995; 4: 461–72.
18. Aboitiz F. Comparative development of the mammalian isocortex and the reptilian dorsal ventricular ridge. Evolutionary consideration. Cereb Cortex 1999; 9: 783–91.
19. Aboitiz F, Montiel J, Marales D. Concha. Evolutionary divergence of the reptilian and mammalian brains: Considerations on connectivity and development Brain Ros Revi 2002; 39:141–53.
20. Hedreen JC, Bacon SJ, Price DL. A modified histochemical technique to visualize acetylcholinesterase cotaining axon. J Histochem Cytochem 1985; 33: 134–40.
21. Goldby F. An experimental evidence of the Cerebral hemispheres of Lacerta viridis J Anat Lond 1937; 71:332–55.
22. Lewis PR, Shute CCD. Selective staining of visceral efferents in the rat brain stem by a modified Koelle technique. Nature, 1959; 183: 1743–44.
23. Hebb C. Cholinergic neurons in vertebrates. Nature. 1961; 192:527–29.
24. Nachmensohn D. Chemical & Molecular basis of nerve activity, physiologically significant features of acetylcholinesterase New York and London. Academic press, 1959.
25. De Robertis E, Pellegraino De Iraldi A, Rodriguez De Lores AG, Salganicaff L. Cholinergic and non cholinergic nerve endings in rat brain. Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J Neurochem 1962; 9: 23–35.
26. Whittaker VP, Michaelson IA, Kirkland RJ. The seperation of synaptic vesicles from disrupted nerve ending particles. Biochem Pharmacol 1963; 2: 300–02.
27. Shute CCD, Lewis PR. The fine localization of Cholinesterase in the hippocampal formation. J Anat. 1965; 99: 938.
28. Bennett EL, Diamond MC, Morimoto H, Herbert M. Acetylcholinesterase activity and weight measures in fifteen brain areas from six lines of rats. J Neurochem 1966; 3: 563–72.
29. Giacobini E. Intracellular distribution of Cholinesterase in the anterior horn cells of rats. Arch Ital Biol 1961; 99: 163–77.
30. Ryan SM, Arnold AP. Evidence for cholinergic participation in the control of bird song: acetylcholinesterase distribution and muscarinic receptor autoradiography in the zebra finch brain. J Comp Neurol 1981; 202: 211–19.
31. Cooksen KK, Hall WS, Heaten JT, Brauth SE. Distribution of choline acetyl transferee and acetylcholinesterese in vocal control nuclei of the budggerigar. J Comp Neurol 1996; 369: 220–35.
(c) Annals of Neurosciences.All Rights Reserved