Annals of Neurosciences, Vol 16, No 4 (2009)
Long term L-DOPA treatment causes production of 6-OHDA in the mouse striatum: Involvement of hydroxyl radical
Corresponding author
K P Mohanakumar, PhD
Tel.: +91-33-24133223
Fax: +91-33-24735197
E-mail:mohankumar@iicb.res.in
Copyright © 2009, The National Academy of Sciences
2009
ABSTRACT
Background:L-DOPA (3,4-dihydroxyphenylalanine), the precursor of dopamine (DA) still remains the mainstay drug for the treatment of Parkinson's disease (PD). L-DOPA is not well regulated at the dopaminergic neurons due to excessive DA production from the antiparkinsonian drug, it has been generally believed that several cytotoxic molecules could be generated in the brain following activation by dopaminomimetics and DA catabolism inhibitors. Purpose: The present study examines the production of 6-OHDA in the striatum of mice treated sub-acutely with L-DOPA, and investigates the possible mechanism of 6-OHDA production from DA. Methods: Male adult Balb/c mice were treated with different doses of L-DOPA/carbidopa combination (25,250 and 500/2.5,25 and 50 mg/kg, p.o) or vehicle for 7 days and 6-OHDA levels were analyzed in the striatum at 1,2,4 and 6 h after the last dose of the drug. Results: We report here that prolonged L-DOPA treatment in mice causes significant elevation in 6-OHDA production from 16 h after the drug treatment, which is dependent on the dose of L-DOPA administered. Dopamine utilization was evident from the reduced levels of its presence in the FAD system following 6-OHDA production. Conclusion: These results point to an inherent hazard of long-term administration of L-DOPA in Parkinson's disease patients.
doi: 10.5214/ans.0972.7531.2009.160406
KEY WORDS
reactive oxygen species,
dopamine oxidation product,
glutathione, catalase,
parkinson's disease
Introduction
L-DOPA (3,4-dihydroxyphenylalanine), the precursor of dopamine (DA) still remains the mainstay drug for the treatment of Parkinson's disease (PD). However, long-term treatment of the drug has called attention to a number of inherent problems, including unpredictable outcome of the treatment, serious side effects and on-off phenomenon, which are in part explained by DA synthesis in brain nuclei other than those contain dopaminergic neurons.1 In addition, it is argued that DA may autoxidize to form highly neurotoxic DA quiñones and semiquinones by reacting with reactive oxygen species, such as superoxide radicals, hydrogen peroxide, and hydroxy I radical (•OH)2–4 that may possess cytotoxic effects. DA can be metabolized by various enzymes, including mitochondrial monoamine oxidase-B,5 which generates cytotoxic H202 as a byproduct.6
One of the DA oxidation products, 6-hydroxydopamine (6-OHDA) is a potent dopaminergic neurotoxin which has been employed to produce experimental PD in rodents.7»10 In vitro, 6-OHDA,11, 12 2-hydroxydopamineand 5-hydroxydopamine»can be synthesized from DA in presence of iron alone or iron and ascorbic acid. Since excessive DA production from the antiparkinsonian drug, L-DOPA is not well regulated at the dopaminergic neurons,1 it has been generally believed that several cytotoxic molecules could be generated in the brain following activation by dopaminomimetics and DA catabolism inhibitors. Supporting this contention, presence of high levels of 6-OHDA have been reported in postmortem PD brains14 and urine samples of PD patients receiving prolonged treatment of L-DOPA.15
Reports on the detection of high levels of 6-OHDA in the brain of experimental animals administered methamphetamine have not been unequivocal. 16–20 More recently Maharaj and colleagues12 have reported production of 6-OHDA in the brain as a result of L-DOPA treatment. One of the basic mechanisms of dopaminergic neurotoxicity by this neurotoxin is increased production of reactive oxygen species including OH in substantia nigra and nucleus caudatus putamen.21,22 Inside the neurons, 6-OHDA can also generate quiñones which are cytotoxic.23
The present study examines the production of 6-OHDA in the striatum of mice treated sub-acutely with L-DOPA, and investigates the possible mechanism of 6-OHDA production from DA.
Methods
Chemicals
L-DOPA tablets containing 10% of carbidopa (Syndopa) were purchased from Sun Pharmaceutical Industries Ltd (Baroda, India), and solubilized in water. Reduced glutathione (GSH), catalase, superoxide dismutase (SOD), ascorbic acid, 6-OHDA, DA HCl, 1-heptane sulfonic acid sodium salt, 2,3- and 2,5-dihydroxybenzoic acid (DHBA), sodium salicylate and ethylenediaminetetraacetic acid disodium salt (EDTA) were purchased from Sigma-Aldrich Co (St. Louis, MO, USA). Octane-1-sulphonic acid, ferrous sulphate, perchloric acid and acetonitrile were obtained from Merck, Mumbai, India. Sodium phosphate monobasic and citric acid were obtained from MP Boimedicals Ine (France).
Animals
Male adult Balb/c mice (20-25 g) were used in the present study. The animals were maintained under standard conditions of 12 h light/dark cycles, 24 ± 2 °C temperature and 60±5% humidity. They were provided with food and water ad libitum. The experimental protocols met the National Guidelines on the »Proper Care and Use of Animals in Laboratory Research»24 and wereapproved by the Animal Ethics Committee of the Institute.
Drug treatment
Adult mice were treated everyday in the morning at 9.00 -12 AM with different doses of L-DOPA/carbidopa combination (25,250 and 500/2.5, 25 and 50 mg/kg, p.o) or vehicle for 7 days and 6-OHDA levels were analyzed in the striatum at 1,2,4 and 6 h after the last dose of the drug.
Analysis of 6-OHDA byHPLC-ECD
Whole brains from mice were dissected out, rinsed in chilled normal saline; blotted dry on ash-free filter paper and the striata were dissected. The tissue was weighed and sonicated in 1 volumeof chilled perchloric acid (0.4 M) containing ascorbic acid (1 mM). It was kept on ice for 30 min and centrifuged at 18,000 x g for 5 min. The supernatant was collected and 10 μl was injected into the HPLC. The mobile phase consisted of 25 mM citric acid, 125 mM sodium phosphate, 100 mg/L EDTA and 30 mg/L octane sulfonic acid, pH adjusted to 2.5 with 85% ortho-phosphoric acid.25 Flow rate was maintained at 0.7 ml/min and the electrochemical detection was performed at +200 mV. The HPLC system consisted of an isocratic pump (BioanalyticaI Systems, West Lafayette, USA), an a m pero metri e detector (Epsilon, Bioanalytical Systems) and C18, ion pair, analytical column (4.6 x 250 mm; Ultrasphere IP; Beckman, USA), with particle size of 5pm and pore size of 80 Á.
HPLC analysis of 2,5- and2,3-DHBA
We used the FAD system containing DA (1 mM), ascorbic acid (1 mM), EDTA (240 pM), ferrous sulfate (200 pM) in 50 mM phosphate buffer at pH 7.2 as described by Slivka and Cohen13 was used in the present study. FAD was incubated with and without GSH (1 mM) or catalase (1000 U) or SOD (1000 U). DA was always added in the last. The reaction mixture was incubated at 37 °C in dark for 120 min. Aliquots (100 /il) were removed at 120 min of incubation, added equal volume of 1 mM of sodium salicylate, kept at room temperature for 5 min, and added 800 pi of 0.4 M perchloric acid containing 1 mM ascorbate. Ten pi of the sa m pie was injected into HPLC system for the detection of 2,5-DHBA and 2,3-DHBA after centrifugation.
The DHBA adducts were analyzed by employing an HPLC-electrochemical detection procedure.26 The composition of the mobile phase was 8.65 mM heptane sulfonic acid, 0.27 mM EDTA, 13% acetonitrile, 0.43% triethylamine and 0.22% orthophosphoric acid. The flow rate was maintained at 0.7 ml/min and the electrochemical detection was performed at +740 mV.
Generation of 6-OHDA from FAD system
We used the FAD system as described by Slivka and Cohen13, with and without GSH at increasing concentrations (0.01, 0.1, 1.0 mM). Similarly, catalase and SOD were used at concentrations of 10, 100 and 1000 Units. DA was always added in the last. The reaction mixture was incubated at 37 °C in dark for 120 min. Aliquotes (100 pi) were removed and reaction was stopped by adding 900 pi of ice-cold 0.4 M perchloric acid containing 1.0 mM ascorbate. 10 pi of this sample was injected into HPLC system for 6-0 H DA analysis. Final results are expressed as pmol/ml. The FAD system incubated for 120 min with 1 mM DA or GSH or catalase (1000 U) or SOD (1000 U) were also assayed for the content of DA employing the HPLC-electrochemical procedure, as described above for 2,5-DHBA and 2,3-DHBA measurements.1'25'26
Statistical analysis
All data were analyzed for significance using Students t-test. Results are given as mean ± SEM. Value of p < 0.05 was considered significant.
Results
L-DOPA treatment generates striatal 6-OHDA in mice
Striata from saline treated, L-DOPA naive mice did not produce any detectable level of 6-OHDA (data not given). Treatment of different doses of L-DOPA (25-500 mg/kg) in mice for 7 days significantly increased the striatal 6-OHDA levels, when analyzed at 1,2,4 and 6 h time interval after 7* dose of the drug. The level was significantly increased with 250 and 500 mg/kg treatments when compared with 25 mg/kg L-DOPA. However, the generation of 6-OHDA by 250 mg/kg L-DOPA was not significantly different from 500 mg/kg. At 1 and 2 h after L-DOPA treatment, the 6-OHDA level was significantly increased by 36% and 37% respectively for 25 mg/kg; and around 2.5 fold increase for both 250 and 500 mg/kg L-DOPA administration when compared with the 25 mg/kg dose effect in mice (Fig 1.).
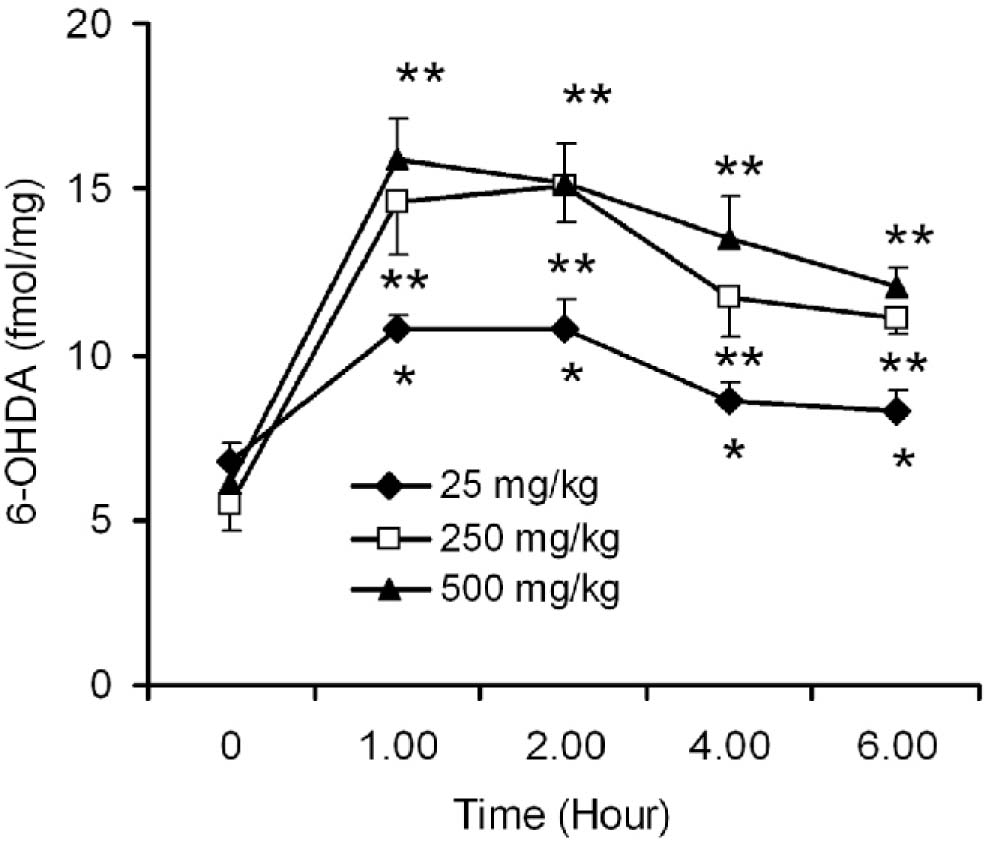
Fig. 1:
Time- and dose-dependent effects of L-DOPA on 6-OHDA production in mouse striatum. Adult Balb/c mice were administered with different doses of L-DOPA (25, 250 and 500 mg/kg, p.o) along with 10% carbidopa for 7 days and the animals were sacrificed at 1, 2, 4 and 6 h after the last dose of the drug. The time point at '0' provides the value of control mice that received no L-DOPA, but saline for 7 days. Striata were dissected and 6-OHDA levels were measured by employing a sensitive HPLC-electrochemical detection procedure. Results are expressed as fmol/mg tissue and mean ± SEM. *p ≤0.05 as compared to control, **P ≤0.05 as compared to 25 mg/kg effects (n = 8).
Effect of GSH, catatase and SOD on OH generation in FAD system
FAD system generated 35.05 ± 10.17 nmol/ml of 2,5-DHBA and 8.06 ± 3.88 nmol/ml of 2,3-DHBA (Fig. 2). Presence of the antioxidant molecule GSH, or the enzymes SOD or catalase in the FAD system caused significant reduction (60%) in the levels of 2,3-DHBA, but not in 2,5-DHBA levels (Fig. 2).
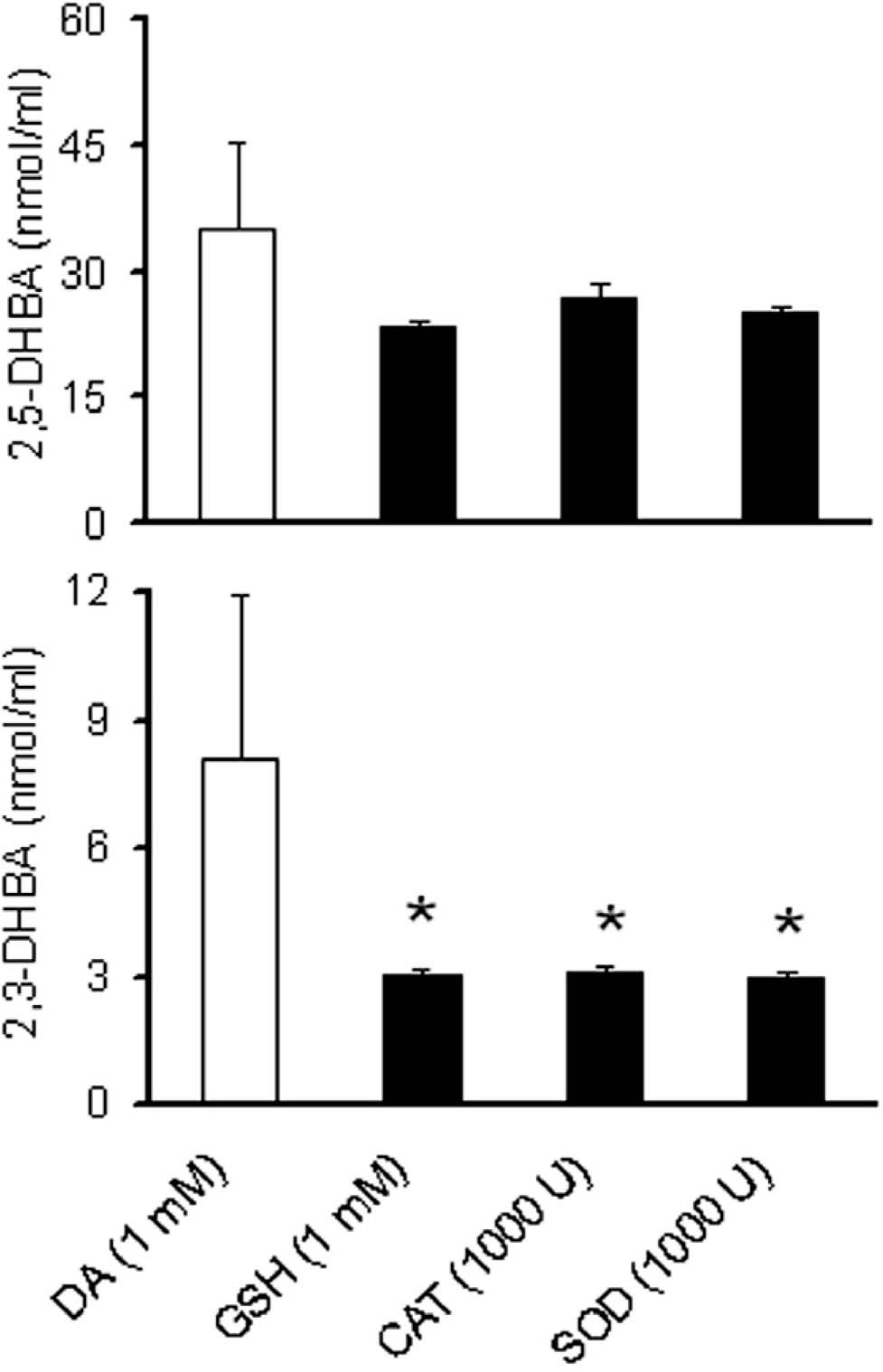
Fig. 2:
Hydroxy I radical generation in FAD system in presence of GSH, catalase and SOD. DA (1 mM) was incubated in a reaction mixture containing 1 mM ascorbate, 240 μM EDTA, 200 μM ferrous sulfate and 50 mM phosphate buffer at pH 7.2 (FAD). GSH (1 mM) was added to this mixture to test the protective effect of this antioxidant on the yield of 2,5-DHBA and 2,3-DHBA. Similarly, catalase (CAT) and SOD were used in the concentration of 1000 U. The reaction mixture was incubated at 37 °C in dark for 120 min. Aliquots (100 μl) were removed at 120 min of incubation, added equal volume of 1 mM of salicylic acid, kept at room temperature for 5 min, and added 200 μl of 0.4 M perchloric acid containing 1 mM ascorbate. Ten pi of the sample was injected into H PLC system for the detection of 2,5-DHBA and 2,3-DHBA, after centrifugation. Results are expressed as nmol/ml of the reaction mixture and are mean ± SEM. *p ≤0.05 as compared to control (n = 8).
DA levels in FAD system after the production of6-OHDA
FAD system which contained 1 m M of DA initially, was left with only 540 ± 80 M of DA after 120 min of incubation. This level of DA was not significantly influenced by GSH (641.85 ± 110), catalase (752 ± 133) or SOD (495 ± 69) (Fig. 3.
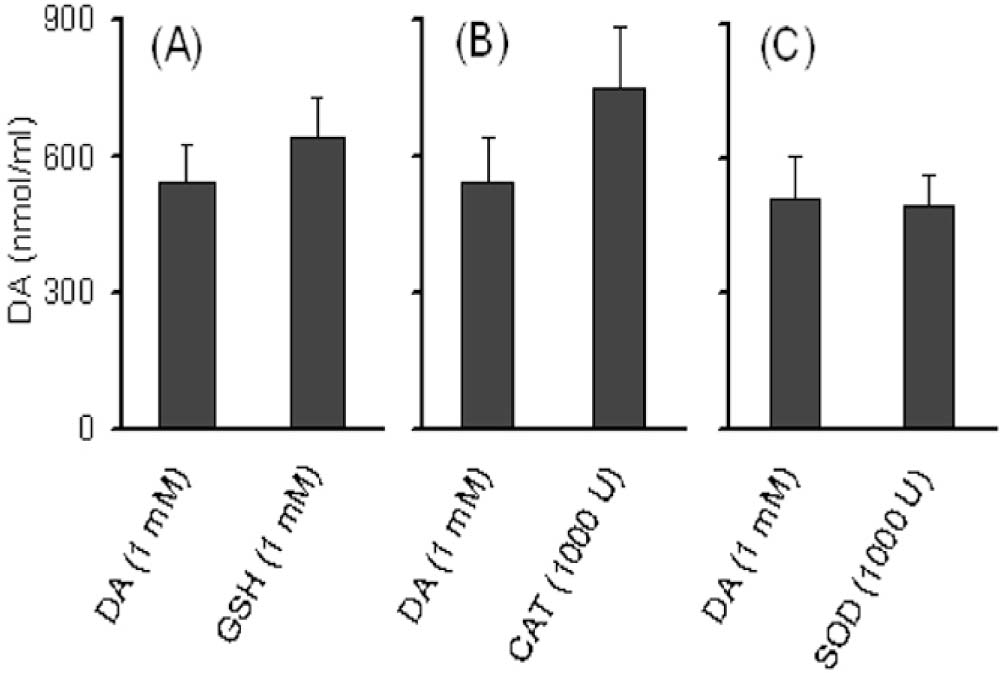
Fig. 3:
Effect of GSH, catalase and SOD on DA levels in FAD system. DA {1 mM) was added in a reaction mixture containing 1 mM ascorbate, 240 μM EDTA, 200 μM ferrous sulfate and 50 mM phosphate buffer at pH 7.2 (FAD). (A) GSH (1 mM) was added to this mixture to test the effect of this antioxidant on DA oxidation. Similarly, (B) catalase (CAT), and (C) SOD were used in the concentration of 1000 U. The reaction mixture was incubated at 37 °C in dark for 120 min. Aliquots (100 μl) were removed and reaction was stopped by addition of 900 μl of ice-cold 0.4 M perchloric acid containing 1.0 mM ascorbate. Ten μl of the sample was injected into H PLC system for the analysis of DA. Results are expressed as nmol/ml of the reaction mixture.
Production of6-OHDA from DA in FAD system
When DA (1.0 mM) was incubated in Fe2+-EDTA-ascorbate system for 120 min, an increased production of 6-OHDA was observed. 6-OHDA production in the FAD system was in the range of 850-1100 pmol/ml (Fig. 4 A-C). GSH at 1 mM, but not at lower concentration attenuated (46%) DA-induced production of 6-OHDA (Fig. 4A). Catalase at 100 U and 1000 U inhibited the production of 6-OHDA in FAD system by 34% and 66% respectively (Fig. 4B). 6-OHDA production was significantly inhibited by SOD, but only at 1000 U, and the effect was the least (30%) amongst the three antioxidants tested (Fig. 4C).
Discussion
A key finding of the present study is the dose and time-dependent production of 6-OHDA in the striatum of mice treated long-term with L-DOPA. Another major finding is the in vitro generation of -OH in the FAD system that has been shown to generate 6-OHDA from DA in a dose dependent manner.25 The third and most important finding of the present study is the attenuation of 6-OHDA by antioxidants molecule, GSH, catalase and SOD. These results strongly suggest the direct involvement of OH in the production of 6-OHDA.

Fig. 4:
Effect of GSH, catalase and SOD on in vitro 6-OHDA generation from FAD system. DA (1 mM) was incubated in a reaction mixture containing 1 mM ascorbate, 240 µ? EDTA, 200 µ? ferrous sulfate and 50 mM phosphate buffer at pH 7.2 (FAD). (A) GSH was added to this mixture in the concentration range of 0.01, 0.1 and 1 mM to test the protective effect of this antioxidant on the yield of 6-OHDA from DA. Similarly, (B) catalase (CAT) and (C) SOD was used in the concentration of 10 U, 100 U and 1000 U. The reaction mixture was incubated at 37 °C in dark for 120 min. Aliquots (100 µ?) were removed and reaction was stopped by addition of 900 µ? of ice-cold 0.4 M perchloric acid containing 1.0 mM ascorbate. 10µ? of the sample was injected into ? PLC system for the analysis of 6-OHDA. The variations in 6-OHDA production without any antioxidants refers to day-to-day variations in the analysis of this hydroxylated amine, in vitro. Results are expressed as pmol/ml of the reaction mixture and are mean ± SEM. *p 50.05 as compared to 6-OHDA produced by 1 mMDA(n = 8).
In PD, free radical generation is considered to be one of the major risk factors.27,28 Dopaminergic neurons are particularly vulnerable to oxidative injury because metabolism of DA produces various molecules that can act as endogenous neurotoxins.29, 30 Auto-oxidation of both L-DOPA or DA produces pro-oxidants like quiñones and semiquinones, and highly reactive oxygen species, which include superoxide radical, hydrogen peroxide, and -OH.2,31 In fact, high doses of DA injected into the striatum has been shown to produce dose-dependent lesions in rat striatum, and this DA-induced neurotoxicity was associated with increased production of DA oxidation products. 32,33 Our finding that L-DOPA treatment increases the 6-OHDA levels in the striatum suggest that OH production results from the increased DA levels in the striatum following L-DOPA treatment. The increased OH and DA level in the striatum provides an excellent environment for hydroxylation of DA to form 6-OHDA in the striatum as it happens in vitro. This is more feasible, since the L-DOPA-induced excess levels of DA in the striatum are not under normal regulatory control of the cell.1More recently, Mosharov ef al 34 have demonstrated, employing patch-clamped cyclic voltametric measurements that extracellular or cytosolic DA which are unregulated, but not the vesicular DA, which are physiologically regulated, are neurotoxic. Combined with this information in the literature and the current findings, it is suggested that chronic exposure to physiologically unregulated extracellular or cytosolic DA would create conducive environment for dopaminergic neurodegeneration, including Huntington's disease.35Interestingly, we have recently demonstrated the higher sensitivity of denervated striatum to produce more 6-OHDA than the partly denervated or innervated striatum in an MPTP model of PD, 25 highlighting the importance of endogenous production of 6-OHDA in the brain following L-DOPA treatment.
Several known antioxidants and other drugs have been used to protect against striatal DA and substantia nigra dopaminergic cell loss in experimental parkinsonism. Aspirin, acetaminophen, salicylic acid, D- or L-deprenyl, melatonin, several of the nitric oxide donors, and nitric oxide synthase inhibitors are among the vast list of clinically used drugs that are known to provide protection by their free radical scavenging action.26,3–7Importantly, drug candidates that are known to exacerbate reactive oxygen species production have been shown to aggravate parkinsonism in animals.4,50 These reports highlight importance of antioxidant molecules in neuroprotective therapeutic strategies in PD. Based on these studies, we examined the effects of endogenous antioxidant molecule, GSH and antioxidant enzymes, catalase and SOD in vitro in FAD on the production of 6-OHDA, and showed that the levels are inhibited by all the antioxidants. While GSH and SOD protect against generation of 6-OHDA in vitro only in higher doses, catalase protected against 6-OHDA levels even with lower enzyme units indicating the involvement of H202 in the production of this neurotoxic molecule. These results suggest that in vivo generation of 6-OHDA could be attenuated byincreased levels of GSH, catalase or SOD. The in vitro and in vivo capacity of melatonin or L-deprenyl to prevent DA-induced generation of 6-OHDA are being reported elsewhere.25,51 The present study has demonstrated the possible neurotoxic effect of L-DOPA administration in PD and suggests the use of the drug that increases the antioxidant levels in order to increase the therapeutic potential. The co-administration of an antioxidant with L-DOPA may increase the half-life of L-DOPA as well as enhance the benefit of other neuroprotective effects that the antioxidant possess.
Acknowledgement
AB is the recipient of senior research fellowship from Council of Scientific and Industrial Research, Government of India.
Competing interests - None, Source of Funding - CSIR
Received Date : 2 September 2009; Revised Date : 4 October 2009
Accepted Date : 14 October 2009
References
1 Borah A, Mohanakumar KP. Long-term L-DOPA treatment causes indiscriminate increase in dopamine levels at the cost of serotonin synthesis in discrete brain regions of rats. Cell Mol Neurobiol 2007; 27:985-996.
2 Graham DC Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quiñones. Mol Pharmacol 1978; 14: 633-643.
3 Rosenberg PA. Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci 1988; 8:2887-2894.
4 Michel PP, Hefti F. Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res 1990; 26: 428-435.
5 Kopin IJ. Catecholamine metabolism: Basic aspects and clinical significance. Pharmacol Rev 1985; 37:333-364.
6 Kostrzewa RM, Kostrzewa JP, Brus R. Neuroprotective and neurotoxic roles of levodopa (L-DOPA) in neurodegenerative disorders relating to Parkinson's disease. Amino Acids 2002; 23:57-63.
7 Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 1970; 24:485-93.
8 Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigrostriatal dopamine system. Acta Physiol Scand Suppl 1971 ; 367:69-93.
9 Sindhu KM, Banerjee R, Senthilkumar KS, et al Rats with unilateral median forebrain bundle, but not striatal or nigral, lesions by the neurotoxins MPP+ or rotenone display differential sensitivity to amphetamine and apomorphine. Pharmacol Biochem Behav 2006;84:321-329.
10 Senthilkumar KS, Saravanan KS, Chandra G, et al Unilateral implantation of dopamine-loaded biodegradable hydrogel in the striatum attenuates motor abnormalities in the 6-hydroxydopamine model of hemi-parkinsonism. Behav Brain Res 2007; 184:11 -18.
11 Napolitano A, Pezzella A, Prota G. New reaction pathways of dopamine under oxidative stress conditions: nonenzymatic iron-assisted conversion to norepinephrine and the neurotoxins 6-hydroxydopamine and 6,7-dihydroxytetrahydroisoquinoline. Chem Res Toxicol 1999; 12:1090-1097.
12 Maharaj H, Sukhdev Maharaj D, Scheepers M, et al L-DOPA administration enhances 6-hydroxydopamine generation. Brain Res 2005; 1063:180-186.
13 Slivka A, Cohen G. Hydroxy I radical attack on dopamine. J Biol Chem 1985;260:15466-15472.
14 Curtius HCWolfensbergerM, SteinmannB, et al Massfragmentography of dopamine and 6-hydroxydopamine. Application to the determination of dopamine in human brain biopsies from the caudate nucleus. J Chromatogr 1974; 99:529-540.
15 Patel S, Sinha A, ParmarD eta/. An update on the role of environmental factors in parkinson's disease. Annals of Neurosciences 2005;12(4):79-86.
16 Seiden LS, Vosmer G. Formation of 6-hydroxydopamine in caudate nucleus of the rat brain after a single large dose of methylamphetamine. Pharmacol Biochem Behav 1984; 21:29-31.
17 Evans J, Cohen G. Catecholamine uptake inhibitors elevate 6- hydroxydopamine in brain after administration of 6-hydroxydopa. Eur J Pharmacol 1993; 232:241 -245.
18 Liao P-C, Kuo Y-M, Chang Y-C, et al Striatal formation of 6- hydroxydopamine in mice treated with pargyline, pyrogallol and methamphetamine. J Neural Trans 2003; 110:487-494.
19 Rollema H, Kuhr WG, Kranenborg G, et al MPP+-induced efflux of dopamine and lactate from rat striatum have similar time courses as shown by in vivo brain dialysis. J Pharmacol Exp Ther 1988; 245: 858-866.
20 Karoum F, Chrapusta SJ, Egan MF, et al Absence of 6-hydroxydopamine in the rat brain after treatment with stimulants and other dopaminergic agents: a mass fragmentographic study. J Neurochem 1993; 61:1369-1375.
21 Dajas-Bailador FA, Martinez-Borges A, Costa G, et al Hydroxyl radical production in the substantia nigra after ?-hydroxydopamine and hypoxia-reoxygenation. Brain Res 1998; 813:18-25.
22 HenzeC, EarIC, Sautter J, et al Reactive oxidative and nitrogen species in the nigrostriatal system following striatal 6-hydroxydopamine lesion in rats. Brain Res 2005; 1052:97-104.
23 Carrasco E, Werner P. Selective destruction of dopaminergic neurons by low concentrations of 6-OHDA and MPP+: protection by acetylsalicylic acid aspirin. Parkinsonism and Related Dis 2002; 8:407-411.
24 INSA. Guidelines For Care and Use of Animals in Scientific Research. Indian National Science Academy Publication. New Delhi 2000; pp1-26.
25 Borah A, Mohanakumar KP. Melatonin inhibits 6-hydroxydopamine production in the brain to protect against experimental parkinsonism in rodents. J Pineal Res 2009; 47:293-300.
26 Muralikrishnan D, Mohanakumar KP. Neuroprotection by bromocriptine against 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J1998; 12:905-912.
27 Adams JD Jr, Odunze IN. Oxygen free radicals and Parkinson's disease. Free Radie Biol Med 1991; 10:161-169.
28 Mohanakumar KP. Molecular events leading to cell death in Parkinson's disease. In: »Brain Disorders Across Life Span: Hopes and Challenges» Ranbaxy Science Foundation Lectures, Series XIV, 2006; pp. 63-75.
29 Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2001; 2:492-501.
30 Maguire-Zeiss KA, Short DW, Federoff HJ. Synuclein, dopamine and oxidative stress: co-conspirators in Parkinson's disease? Brain Res Mol Brain Res 2005; 134:18-23.
31 Tse DC, McCreery RL, Adams RN. Potential oxidative pathways of brain catecholamines. J Med Chem 1976; 19:37-40.
32 Filloux F, Townsend JJ. Pre- and postsynaptic neurotoxic effects of dopamine demonstrated by ¡ntrastriatal injection. Exp Neurol 1993;119:79-88.
33 Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA 1996;93:1956-1961.
34 Mosharov EV, Larsen KE, Kan ter E, et al Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009; 62:218-229.
35 Pandey M, Borah A, Varghese M, et al Striatal dopamine level influences oxidative stress mediated neurodegeneration in 3-nitropropionic acid-induced Huntington's disease. Neurochem Int2009; 55:431-4377.
36 Wu RM, Mohanakumar KP, Murphy DL, et al Antioxidant mechanism and protection of nigral neurons against MPP+ toxicity by deprenyl (selegiline). Ann NY Acad Sci 1994; 738:214-221.
37 Mohanakumar KP, Muralikrishnan D. Thomas B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Brain Res 2000; 864: 281-290.
38 Dabbeni-Sala F, Di Santo S, Franceschini D, et al Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J 2001 ; 15:164-170.
39 Leret ML, San Millan JA, Fabre E, et al Deprenyl protects from MPTP- induced Parkinson-like syndrome and glutathione oxidation in rat striatum. Toxicology 2002; 170:165-171.
40 Sairam K, Saravanan KS, Banerjee R,
41 Muralikrishnan D, Samantaray S, Mohanakumar KP. D-deprenyl protects nigrostriatal neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity. Synapse 2003;50:7-13.
42 Maharaj DS, Saravanan KS, Maharaj H, et al Acetaminophen and aspirin inhibit superoxide anion generation and lipid peroxidation, and protect against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats. Neurochem Int 2004;44: 355-360.
43 Thomas B, Mohanakumar KP. Melatonin protects against oxidative stress caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the mouse nigrostriatum. J Pineal Res 2004; 36:25-32.
44 Thomas B, Saravanan KS, Mohanakumar KP. In vitro and in vivo evidences that antioxidant action contributes to the neuroprotective effects of the neuronal nitric oxide synthase and monoamine oxidase-B inhibitor, 7-nitroindazole. Neurochem Int 2008; 52:990-1001.
45 Saravanan KS, Sindhu KM, Senthilkumar KS, et al L-Deprenyl protects against rotenone-induced, oxidative stress-mediated dopaminergic neurodegeneration in rats. Neurochem Int 2006; 49:28-40.
46 Saravanan KS, Sindhu KM, Mohanakumar KP. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res 2007; 42:247-53.
47 Banerjee R, Saravanan KS, Thomas B, et al Evidence for hydroxy I radical scavenging action of nitric oxide donors in the protection against 1-methyl-4-phenylpyridinium-induced neurotoxicity in rats. Neurochem Res 2008; 33:985-995.
48 KnaryanVH, Samantaray S, GaloyanAA, et al Asynthetic human proline- rich-polypeptide enhances hydroxyl radical generation and fails to protect dopaminergic neurons against 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced toxicity in mice. Neurosci Lett 2005;375:187-191.
49 Knaryan VH, Samantaray S, Varghese M, et al Synthetic bovine proline- rich-polypeptides generate hydroxyl radicals and fail to protect dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity in mice. Neuropeptides 2006; 40:291-298.
50 Navneet AK, Appukuttan TA, Pandey M, et al Taurine fails to protect against 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion in mice. Amino Acids 2008; 35: 457-461.
51 Borah A, Mohanakumar KP. Denevated, but not innervated striatum of parkinsonian rats is sensitive to 6-hydroxydopamine production in vivo following prolonged L-DOPA administration. Neurochem Int 2009; doi: 10.1016/J neuint 2009.11.008.
(c) Annals of Neurosciences.All Rights Reserved