Annals of Neurosciences, Vol 15, No 4 (2008)
Annals of Neurosciences, Volume 15, Issue 4 (October), 2008
ACETYLCHOLINESTERASE :A VERSATILE ENZYME OF NERVOUS SYSTEM
Corrosponding Author
Dr UC Srivastava
Professor of Zoology
Department of Zoology, University of Allahabad
Allahabad, India – 211 002
Phone : 93351 20178
E-mail :
(Date received: 08.09.2008)
Abstract
Acetylcholinesterase (AChE) terminates the neurotransmission at cholinergic synapses by splitting the neurotransmitter acetylcholine. The nature and didtribution of the enzyme has extensively been studied in many invertebrates and vertebrates including human, histochemically and biochemically. The enzyme demonstrates a high degree of variability in distribution with its notable presense in nonneuronal tissues also, which provides pertinent theme to investigate its nonclassical role.
Recently a lot of information has come out regarding its dynamic structure, gene expression, its role in neuronal morphogenesis and synaptogenesis. The significance of AChE stems from the fact that it is the target of drugs designed to treat myasthenia gravis, glaucoma, alzheimer's disease etc.
Keeping in view, above mentioned facts a thorough review has been made in the present article regarding its biochemistry, structural dynamics, wide distribution, isoforms and its implications in neurodegenerative disorders.
Key words : Acetylcholinesterase, Cholinergic, Molecular forms, Neurodegenerative disorders, Alzheimer's disease.
Introductions
Acetylcholinesterase ( AChE) is one of the most efficient enzymes of nervous system which is concentrated at the cholinergic synapses and at neuromuscular synapses where it rapidly hydrolyses the neurotransmitter acetylcholine (Ach) in to choline and acetate thus playing an essential role in cholinergic neurotransmission.
The term acetylcholinesterase was introduced in 1949 by Augustintion and Nachmansohn for specific cholinesterase capable of hydrolyzing acetylcholine faster than other esterases. In 1964 the commission of enzymology recommended the name “Acetylcholinesterase” (Acetylcholine Acetyl hydrolase; 3.1.1.7) for a true and specific cholinesterase.
The distribution of the enzyme in the central and peripheral nerve tissues of different vertebrates demonstrates a high range of variation (1–13). It has been noted to be localized in non neuronal tissues and Glial cells also (14, 15). The enzyme also exhibits molecular diversity with its six different molecular forms and structural dynamics which facilitates its affinity and action with various legends (16–17). In addition, AChE is considerd to play several non classical roles independent of its catalytic function i.e. hydrolysis of Ach. These classical and non classical roles of AChE illustrate adequacy about its wide occurrence in neuronal and non neuronal tissues (18–20).
The importance of AChE in body homeostasis is underscored by the fact that they are the targets of some of the most potent toxins including insecticides, snake venom and chemical weapons (21).
Therefore such a wide distribution and various functions, molecular forms, structural dynamics etc. of AChE provide adequate base to recall it a versatile enzyme, a detailed knowledge of which, will help design specific drugs to combat various neurodegenerative diseases associated with this enzyme.
Biochemistry and structural dynamics
The three dimensional structure of AChE was first determined in by Joel Sussman et.al. in1991, using the protein from T. californica (22). The structures of the catalytic domains of the AChEs from species as T. californica, mouse and human are quite similar (fig.1) (23, 24).
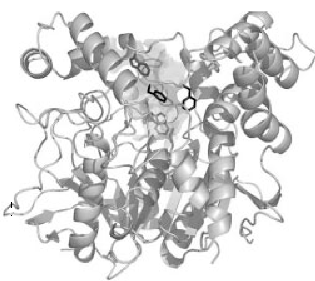
Figure 1. Three-dimensional ribbon diagram of Torpedo Californica acetylcholinesterase.
Acetylcholinesterase is a serine hydrolase belonging to the family of type B corboxylesterase within higher eukaryotes. It is an ellipsoidal molecule approximately 45× 60×65 A0, which consists of 12 stranded mixed beta sheet surrounded by 14 alpha helices. (22). It is a monomer in its natural state with a molecular weight around 60,000 and forms aggregates which continues to produce catalytic activity.
The active site is composed of two subsites, esteric subsite having catalytic triad (Ser-His-Glu) and peripheral binding anionic subsite (PAS) that accommodates the positive quaternary pole of Ach. ( Fig. 2a)
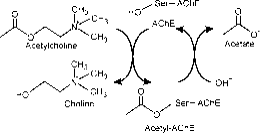
Figure 2a: Different sites of AChE; Catalytic site is located in a deep gorge.
The esteric subsite contains the catalytic machinery of the enzyme, a triad of Ser 200, His-440 and Glu-327 (numbers designate the sequence of amino acids in the polypeptide chain). This triad is similar to other serine proteases except that the triad is first to show Glu as the third member as opposed to Asp. In addition the triad is of opposite handedness to that of the other proteases. The anionic subsite is defined by Trp 84, Phe 330, Phe 331. Its role is to orient charged part of substrate that enters the active site. This is mainly carried out by Trp. 84 (22).
The aromatic gorge in the protein is approximately 20 angstrom deep and penetrates half way the enzyme. The active site lies at the base of this gorge only 4 angstrom above the base. Aromatic gorge is more appropriate term because 40% of its lining is composed of 14 aromatic residues which are highly conserved from different species of AChE (25).
Peripheral anionic site has the ability to bind to many different types of ligands by this it affects the confirmation of active site. Six residues stabilize the activity within site. Trp 286, Ty r 72, Tyr 124, Glu 285, Asp 74, Tyr 341 located on the opposite site of gorge entrance. This array of residues provides flexibility which accommodates many ligands and also implies their confirmation mobility. AChE catalyses the hydrolysis of AChE in the following way (Fig. 2b):
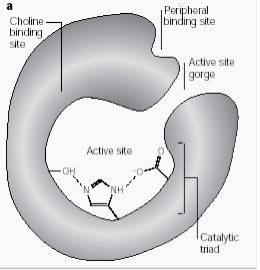
Figure 2b: Enzyme substrate reaction illustrating acetylcholine hydrolysis.
The molecular dynamics of AChE is maintained through cross talk mechanism in which interaction between two subsites takes place particularly between Trp 286, Trp 86 residues. When Trp 286 is bonded on periphery it affects Trp 86 in the active site and causes distinct confirmation site. Asp 74 also plays role in allosteric modulation of enzyme. The sensitivity of these residues and plasticity of the active site are probably the result of evolutionary design aimed to confer optimal activity under a wide veriety of conditions (26.)
Occurrence
AChE enzyme is present in high concentration in all types of conducting tissue, nerve and muscle, central and peripheral tissues, motor and sensory fibres, sympathetic and parasympathetic so called cholinergic and noncholinergic fibres and all the regions where cell bodies and junctions are located, the concentration of the enzyme is high(27). With histochemical methods, AChE is generally demonstrated in neuronal structures: perikarya, surface membranes, axons, dendrites and synaptic regions 4, 15, 28–30). The activity of AChE is higher in motor neurons than in sensory neurons (4, 15, 31). Acetylcholinesterase in nervous tissues is generally acknowledged to represent neurons which contain and presumably released acetylcholine as a neurotransmitter (32).
Early studies of the site of synthesis of AChE within nerve tissue indicated an origin within the cell body of the neuron (33–35). The enzyme has been found to be associated with the endoplasmic reticulum of the cell body and the Golgi apparatus (8, 36). AChE has also been associated in brain subcellular fractions with microsomal and crude mitochondrial fractions (2, 37). Further fractionation of the crude mitochondrial fraction from brain tissue showed high levels of AChE activity in the synaptosomal membrane fraction (6).
The bulk of AChE was found to be localized in the axons and to be associated with axonal membrane (9). Cholinesterase activity limited to axons, has been observed on axonal vesicles of all unmyelinated and some myelinated nerve fibres (38). Myelinated nerve fibres in general have lower concentration than the so called unmyelinated and the white matter contains much less AChE than does the gray matter (27). In isolated motoneurons, AChE activity is about ten times greater in the cytoplasm, dendrites and axons than in the nucleoplasm and no activity is present in the nucleolus (39). Glial cells have been shown to contain AChE in varying concentrations (14, 15)
Classical and Non Classical Roles
The entire course of cholinergic transmission particularly at the neuromuscular junction, namely the release of Ach, its diffusion across the synaptic cleft, its reversible interaction with nicotinic Ach receptor and finally hydrolysis by AChE occurs within a few milliseconds. The whole process is tightly integrated both spatially and temporally (40). In accordance with its classical role, its turn over time is 150 usec, which is equivalent to the hydrolysis of 5000 Ach molecules/molecule of enzyme/sec. It hydrolyses substrate so rapidly that the concentration around the enzyme molecule is depleted relative to its concentration in bulk solution.
For over twenty years, AChE is supposed to play other roles, in addition to its classical role in terminating synaptic transmission. Such non classical functions of AChE might involve the hydrolysis of Ach in a non synaptic context. The 26 residue C terminal peptide ARP ( acetylcholinesterase read through peptide) of human AChE which is produced when the R splice variant is induced by stress has been reported to modulate haematopoitic differentiation (41).
Various in vivo and in vitero studies in the central nervous system suggested that developmentally regulated AChE enzyme plays a role in non cholinergic function like morphometric processes, cell differentiation and synaptogenesis along nervous system (20).There are ample studies that suggest that one of the non classical roles of AChE may be as an adhesion protein involved in synaptic development and maintenance (42). Certain other evidences implicate AChE as bone matrix protein, and it has recently been shown to interact with the basement protein laminin (43, 44).
Several studies have proved that AChE is involved in neurite growth. Forms of AChE that hydrolyse Ach but lacked the corboxy-terminal (responsible for neuritogenic activities), neuritic domains failed to enhance neuritic growth demonstrating the independence of the catylitic and neuritogenic activity from each other (19). AChE has also been proposed to play multiple roles in embryogenesis. Zebra fish provide an excellent system to investigate the invivo roles of AChE during embryogenesis. A missence mutation in the zebra fish ache gene was identified that abolishes Ach hydrolysis but leaves the neuritogenic corboxy-terminal intact (45). In case the ache mutant embryos were also reported to have defects in muscle fibre development and primary sensory neuron survival and dendritic growth. These defects were collectively interpreted to support non classical roles of AChE.
There are ample evidences in support of its role in the hydrolysis of substance P, met, leu-enkephaline and degradation of other neuropeptides as well (46, 47).
AChE has also been proposed to function in heart morphogenesis. AChE activity is detected in embryonic rat and chicken hearts before innervation (48, 49). Though its role in embryonic heart is unclaear, AChE has been proposed to regulate an embryonic impulse conduction system (50). However additional functions are required to further explore the role of AChE in heart function.
AChE has been shown to accelerate the assembly of αβ peptide in to amyloid fibrils probably through interaction at the peripheral site (51–53).It has also been shown that AChE / αβ complexes display enhanced neurotoxicity compared with fibils containing only αβ (54).
In a pharmacological context the studies revealed that non classical activities of AChE appear to converge on the peripheral anionic site. It could be envisaged thus, that new categories of AChE inhibitors will be developed in future.
Molecular diversity
The enzyme AChE occurs in an array of molecular forms differing in both , quaternary structure and the mode of anchoring within the synapse generated by the alternate splicing of the C terminal exon of a single gene, followed by post translational modification (55, 56). Acetylcholinesterase (AChE) exists into six different forms (isoenzymes) viz globular monomer (G1), dimer (G2) and tetramer (G4); tailed tetramer (A4), double tailed tetramer (A8) and triple tailed tetramer (A12) ( fig.3). The most rational terminology based on this information is that developed by Bon et al. (1982). In their system the globular forms are designed by “G” and tailed forms by “A”.
Monomer and disulfide linked dimmer forms may be soluble or attached to a membrane by a glycophospholipid. The tetramer forms may be soluble, lipid linked to membrane or attached to a collagen triple helix. Monomer forms are generally incorporated into synapses of central nervous system of vertebrates, while at the neuromuscular junctions three catalytic subunits of tetramers are attached to a triple helical collagen tail which anchors then to the basal lamina within synaptic cleft.
The expression of AChE molecular forms, in Torpedo, was observed to be tissue-specific suggesting that polymorphism exhibited by AChE is dependant on a number of factors including
[1] amount of Ach released
[2] size of the synaptic space and/or
[3] temporal course of the physiological stimulation.(58)
Regulation of AChE expression in neurons
In recent years there have been extensive studies to unravel the molecular mechanisms and pathways involved in AChE expression and localization in neuronal and non neuronal tissues. AChE is highly expressed during neuronal differentiation. It has been observed that the AChE – 3′ untranslated region (UTR) contains an AU rich element (ARE) that interacts directly with RNA binding protein ‘HuD’ resulting in the abundance of AChE transcripts in neuronal cells (59). These findings show the importance of post transcriptional mechanisms in regulating AChE expression in differentiating neurons and implicate ‘HuD’ as a key trans acting factor in these events. However in addition several other regulatory factores govern the stability, localization and translation of AChE transcripts. Many cis acting elements and trans acting factors and proteins interact with AChE – 3′-UTR. (59).
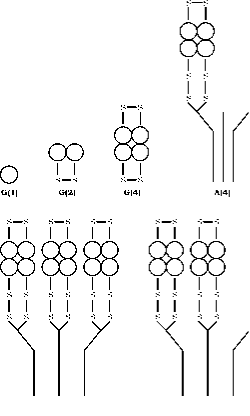
Figure 3. Quarternary structure of the six main forms of AChE. Globular forms are represented by G and asymmetric forms by A according to the scheme of Bon et. al. (16). The location of disulphide bridges and the mode of binding to the collagen tail are diagrammed after the model constructed by Anglister and Silman.(57)
AChE in neurodegenerative and neuromuscular disorders :
Acetylchlinesterase has been directly linked to certain neuromuscular disorders like myasthenia gravis,glaucoma (60),and most recently to alleviate the cholinergic deficiency associated with alzheimer's disease (61).
Clinically, moderate inhibition of AChE is effective in the treatmen of these diseases to prolong the effect of ACh on the receptor Such treatment is desirable either if there is reduced concentration of ACh, as in the case of Alzheimer's disease, or if there are fewer receptors, as in the case of myasthenia gravis. Currently U.S. FDA-approved inhibitors for treatment of alzheimer's disease are E2020 (donepezil, Aricept), tacrine (Cognex), rivastigmine (Exelon), and galantamine (Reminyl)(62). Inhibitors for treatmen of myasthenia gravis include pyridostigmine (Mestinon) and neostigmine (Prostigmine (63). However, overwhelming inhibition of AChE, particularly by covalent bonding to the active site serine, is invariably lethal. Hence, AChE is a prime target for naturally occurring toxins such as the snake venom fasciculin II, pesticides such as parathion and malathion, and chemical warfare agents such as sarin, tabun, and VX (64)
Conclusions
In the present article, we first surveyed the recent outcomes related to the biochemistry and molecular structure of AChE which provides the unique enzyme, flexibility to encounter diverse legends and also to exhibit high turn over rate. We have also illustrated its wide histochemical distribution pattern in the neuronal and non neuronal tissues which can be further correlated to its classical and non classical roles that has been reviewed. Further studies about the regulation of AChE expression, which unravel the importance of these post transcriptional events in vivo are of utmost significance because ultimately these could lead to the design of additional therapeutic strategies aimed at promoting neuronal regeneration and survival. The recent developments in AChE biology particularly its implications in certain neurodegenerative disorders are of key interest that has been reviewed briefly. This research is resulting in the production of new classes of AChE inhibitors targeted against its PAS (peripheral anionic sites), which might play dual roles in the context of Alzheimer's disease by simultaneously inhibiting acetylcholine hydrolysis and retarding assembly of theá â peptide to amyloid fibre.
Abbreviations
AChE – acetylcholinesterase; ARP – acetylcholinesterase read through peptide; Ach – acetycholine; UTR – untranslated region; ARE – AU rich element; PAS – peripheral anionic site; Glu – glutamic acid; His – histidine; Ser – serine; Asp – aspartic acid; Trp – tryptophan; Tyr – tyrosine; Phe – phenyl alanine.
References
1. Boell J, Nechmansohn D. Electron microscopic localization of cholinesterase, by a copper-lead-thiocholine technique. J Neurochem 1966; 3: 1345 – 49.
2. Aldridge WN, Johnson MK. Cholinesterase, succinic dehydrogenase, nucleic acids, esterase and glutathione reductase in subcellular fractions from rat brain. J Biochem1959; 73:270–76.
3. Gerebtzoff MA. Cholinesterases. A histochemical Contribution to the solution of some functional problems. pergemon press, London, 1959.
4. Chacho LW, Cerf JA. Histochemical localization of cholinesterase in the amphibian spinal cord and alterations following ventral root section. J Anat 1960; 94:74–81.
5. Hebb C. Cholinergic neurons in vertebrates. Nature (London) 1961; 192:527–29.
6. De Robertis E, Pellegraino De Iraldi A, Rodriguez De Lores AG, salganicoff L. Cholinergic and noncholinergie nerve endings in rat brain. Isolation and subcellular distribution of acetylcholine and acetylcholinesterase. J Neurochem 1962; 9:23–35.
[%1107. Lewis PR, Shute CCD. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the hippocampus. J physiol 1964; 172:9–10.
8. Shute CCD, Lewis PR. The fine localization of cholinesterase in the hippocampal formation. J Ant 1965; 99: 938.
9. Papp M, Bozsik G. Comparison of the cholinesterase activity in the reticular formation of the lower brain stem of cat & rabbit. J Neurochem 1966; 12: 697–703
10. Bennett EL, Diamond MC, Morimoto H and Herbert M. Acetylcholinesterase activity and weight measures in fifteen brain areas from six lines of rats. J Neurochem 1966; 3 : 563–72.
11. Phillis JW. Acetylcholinesterase in the feline cerebellum.J Neurochem 1968 ; 15:611–18.
12. Tripathi A, Srivastva UC. Histoenzymological distribution of acetylcholinesterase in the cerebral hemispheres of Indian wall lizard, Hemidactylus sflaviviridis Ann Neuro Sci 2007; 14 : 64 -71.
13. Bhasker KS, Joy KP. Acetylcholinesterase-positive intrapineal neuronal system in the palm squirrel Funambulus pennati. Biol struct Morphog 1989; 2: 7–11.
14. Brightman MW, Alberr RW. Species differences in the distribution of extra neuronal cholinesterase within the vertebrate central nervous system. J Neurochem 1959; 3:244–50.
15. Koelle GB. The histochemical localization of cholinesterases in the central nervous system of the rat. J comp Neurol 1954; 100:211–35.
16. Bon S. Molecular forms of acetylcholinesterase in developing Torpedo embryo. Neuro chem 1982; 4 : 577 – 85.
17. Shen T, Tai K, Henchman RH, Mc Cammon HA. Molecuolar dynamics of acetylcholinesterase. Acc chem Res 2002; 35: 332 – 40.
18. Soreq H, seidman S. Acetylcholinesterase – new roles for an old actor. Nat Rev Neurosci 2001; 2 : 294 -302.
19. Downes GB, Granto M. Acetylcholinesterase function is dispensable for sensary neurite growth but is critical for neuromuscular synapse stability. Dev Biol 2004; 270 : 232 – 45.
20. Silman I, Sussman JL. acetylcholinesterase : Classical and non-classical functions and pharmacology. Curr opin pharmacol 2005; 5 : 293 -302.
21. Silman I, Sussman JL. Structural studies on cholinesterases. In cholinesterases and cholinesterase inhibitors. (Ed) Giacobini E. London. Mortin Dunitz 2000 : 9 – 25.
22. Sussman JL, Harel M, Frolow F, et al. Atomic structure of acetylcholinesterase from Torpedo californica : a prototype acetylcholine binding protein. Science 1991; 253 : 872 – 81.
23. Bourne Y, Taylor P, Marchot P. Acetylcholinesterase inhibition by fasciculin: Crystal tructure of the complex. Cell 1995; 83 : 503–12
24. Kryger G, Harel M, Giles K et al. Structures of recombinant native and E202Q mutant human acetylcholinesterase complexed with the snake-venom toxin fasciculin-II. Acta Crystallogr. 2000; 56 : 1385–94.
25. Harel M, Schalk I, Ehret Sabatie L et al. Proc. Natl Acad Sci USA 1993; 90: 9031 – 36.
26. Ordentlich A, Bark D, Kronmen C, Ariel N, Segall Y, Velan B. J Biochem 1995; 269; 11233 – 42.
27. Nachmensohn D. Chemical and molecular basis of nerve activity. Physiologically significant features of acetylcholinesterase & Academic press, New York and London 1959.
28. Bhatt DK, Tewari HB. Histochemical mapping of acetylcholinestarase and butyrylcholinesterase in the medulla oblongata and pons of squirrel J Neurosci Res 1978; 3:419–39.
29. Soderholm. Histochemical localization of esterases, phosphatases and tetrazolium reductases in the motor neurons of the spinal cord of the rat and the effect of nerve division. Aceta physiol. Scand 1965; 65: 3–60.
30. Tripathi A, Srivastava UC. Histochemical mapping of acetycholinesterase in the mesencephalon of Indian wall lizard, Hemidactylus flaviviridis. J Appl Biosc 2007; 3: 145–9.
31. Srivastava UC, Tripathi A. Distributive pattern of acetylcholinesterase in the medulla oblongata of Indian house wall lizard, Hemidactylus flaviviridis. Natl Acad Sci Lett 2007; 30: 325 – 33.
32. La Bella FS, Shin S. Estimation of cholinesterase and choline acetyltransferase in bovine anterior pituitary, posterior pituitary and pineal body. J Neuro Chem 1968; 15 : 335–42.
33. Lewis PR, Hughes AFW. Metabolism of the nervous system. D Richter, (Eds), Pergamon Press, London 1957: 511.
34. Fukuda T, Koella GB. The cytological localization of intracellular neuronal acetycholineserase. J Biophys Biochem cytol 1959 ; 5 : 433 -40.
35. Filogamo G, Candiollo L. Observations on the behaviour of acetylcholinesterase in the nerve cells of the spinal reflex arc, after section of the peripheral nerves. Experimental investigations in Lepus cuniculus. L Acta anat 1962; 51 : 273–91.
36. Eranko O, Rechardt L, Hanninen L. Rates of regeneration of acetylcholinesterase in rat brain subcellular fractions following DFP inhibition. J Neurochem 1970; 17 : 705–07.
37. Mc Caman RE, Rodriguez G, De Robertis E. Rates of regeneration of acetylcholinesterase in rat brain sub cellular fractions following DFP inhibition. J Neurochem 1970; 12 : 927.
38. Schlaepfer WW, Torack RM. The ultra structural localization of Cholinesterase activity in the sciatic nerve of the rat. J Histochem Cytochem 1966; 14: 369–77.
39. Giacobini E. Intracellular distribution of cholinesterase in the anterior horn cells of rats. Arch Ital Biol 1961; 99: 163–77.
40. Anglister L, stiles JR, Salpeter MM. Acetylcholinesterase density and turn over number at frog neuromuscular junction, with modeling of their role in synaptic function. Neuron 1994; 12 : 783 – 94.
41. Deutsch VR, Pick M, Perry C, et al. The stress associated acetylcholinesterase variant AChE – R is expressed in human CD 34 (+) hematopoietic progenitors and its C – terminal peptide ARP promotes their proliferation. Exp Hematol 2002; 30 : 1153 – 61.
42. Zeev – Ben – Marcle hai T, Rydberg EH, Solomon A, et al. The intra cellular domain of the Drosophila cholinesterase. Like neural adhesion protein, gliotactin, is natively unfolded. Protein, 2003; 53 : 750 -67.
43. Genever PG, Birch MA, Brown E, Skerry TM. Osteoblast derived acetylcholinesterase : a novel mediator of cell matrix interactions in bone ? Bone 1999; 24 : 297 -303.
44. Paraoanu LE, Layer PG. Mouse acetylcholinesterase interacts in yeast with the extra cellular matrix component laminin – 1 beta. FEBS Lett 2004; 576 : 161 – 64.
45. Hanneman E, Wester-field M. Early expression of acetylcholinesterase activity in functionally distinct neurons of the Zebra-fish. J Comp Neurol 1989; 284 : 350 – 61,
46. Chubb IW, Hodgson AJ, White, CH Acetylcholinesterase hydrolyses substance P.Neuroscience 1980 ; 5 :2065–72.
47. Chubb IW, Ranieri, E Hodgson, AJ, White, GH. The hydrolysis of Leu and Met-enkephalin by acetylcholinesterase. Neuroscilett Suppl 1982 ; 8 :539.
48. Lamers WH, Karts chot A, Los JA, Moorman A F. Acetylcholinesterase in prenatal rat heart : a member for the early development of the cardiac conductive tissue? Anat Rec 1987; 217 : 361 – 70.
49. Lamers WH, Geerts WJ, Moorman A F. Distribution pattern of acetylcholinesterase in early embryonic chicken hearts. Anat Rec 1990; 228 : 297 – 305.
50. Nakamura T, Ikedia T, Shimokaura I, et al. Distribution of acetylcholinesterase actively in the rat embryonic heart with reference to HNK 1 immunoreactivity in the conduction tissue. Anat Embryol 1994; 190 : 367 – 73.
51. Inestrosq NC, Alverez A, Perez CA, et al. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils : possible role of the peripheral site of the enzyme. Neuron 1996; 16 : 881 – 91.
52. Reyes AE, Perez DR, Alvarez A, et al. A monoclonal antibody agains acetylcholinesterase inhibits the formation of amyloid fibrils induced by the enzyme. Biochem Biophys Res Commun 1997; 232 : 652 – 55.
53. Bartolini M, Bertucci C, Cavrini V, Andrisano V. Beta amyloid aggregation induced by human acetylcholinesterase inhibition studies. Biochem Pharmacol 2003; 65 : 407 – 16.
54. Alvarez A, Alarcon R, Opazo C, et al. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemica properties of the enzyme and increase the neurotoxicity of alzheimr's fibries J Neurosci 1998; 18 : 3213 – 23.
55. Silman I, Futerman AH. Modes of attachment of acetylcholinesterase to the surface membrane. Eur J Biochem 1987; 170 : 11 -22.
56. Massoulie J. The origin of the molecular diversity and functiona anchoring of cholinesterase. Neurosignals 2002; 11 : 130 – 43.
57. Anglister L, Silman I. Molecular structure of elongated forms of electrica acetylcholinesterase. J Moloc Biol 1978; 125 : 293 – 311.
58. Massoulie H, Razzementi L, Bon S, Krojei E, Vakkette FM. Molecula and cellular biology of cholinesterase. Prog Neurobiol. 1993; 41 : 31 – 91.
59. Furry HD, Belanger G, Bizzozera NP and Jasmin BJ. Post transcription and regulation of acetylcholinesterase m-RNAs in nerve growth factor treated PC 12 cells by the RNA building proteins HuD. J Biolchem 2003; 278 : 5710 – 17.
60. Taylor P: Anticholinesterase agents. In The Pharmalogical Basis of Therapeutics. (Eds) Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, New York. McGraw-Hill; 1996 : 161 – 76.
61. Giacobini E : Cholinesterase inhibitors : from the calabar bean to Alzheirmer therapy. In Cholinesterases and Cholinesterase inhibitors. Edited by Giacobini E. London : Martin Dunitz; 2000 : 181 – 226.
62. Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer's disease. Drugs 2001; 61: 41–52.
63. Wittbrodt E T. Drugs and myasthenia gravis: an update. Arch Intern Med 1997; 157: 399–408.
64. Taylor P. In The Pharmacological Basis of Therapeutics, (Eds).; New York. McGraw-Hill:, 1996; 161–176.
(c) Annals of Neurosciences.All Rights Reserved