Annals of Neurosciences, Vol 17, No 1 (2010)
Annals of Neurosciences, Volume 17, Number 1, January 2010
Neuroprotective action of Piper longum against MPTP-induced changes in mouse brain
KEYWORDS
Parkinson’s disease
MPTP
Piper longum
Histopathology and
Biochemical analysis
Corresponding Author
Vanisree Arambakkam Janardhanam
Tel.: +91-44-26493652
Fax: +91-44-22352494
E-mail: vanielango@gmail.com
ABSTRACT
Background: Parkinson’s disease is a neurodegenerative disorder characterized by the progressive and selective loss of dopaminergic neurons. Purpose: The study was designed with the aim of investigating the effect of Piper longum in experimental model of Parkinson’s disease. Methods: Swiss albino mice were divided into the following 4 groups: group I received vehicle saline (0.25 ml); group II was treated with 20 mg/kg body weight of MPTP(2 dose with 2 h intervals); group III received MPTP along with 50 mg/kg body weight of Piper longum and group IV received 50 mg/kg body weight Piper longum. Results: MPTP induced mice showed vascular degeneration and cytoplasmic vacuolation in the brain tissue. In group II the specific activities of superoxide dismutase and, catalase were increased with increase in lipid peroxidation along with reduction in the activities of adenosine tri phosphatase. Conclusion: The oxidative stress and related biochemical alteration by MPTP were altered in Piper longum treatment. However, further studies are needed to assess the precise mechanism to support the clinical use of the plant as a source of antiparkinsonian drug.
doi: 10.5214/ans.0972.7531.2010.170105
Introduction
Parkinson’s disease is one of the most common neurodegenerative disorders, characterized by a relatively selective degeneration of dopaminergic neurons in the substantia nigra pars compacta.1-3 However, pathological cell death within this nucleus is not uniform. The symptoms include resting tremor, bradykinesia, postural instability, and rigidity.4-6 1, methy, 4 phenyl 1,2,3,6 tetra hydro pyridine (MPTP) is a potent neurotoxin that induces Parkinson’s disease in various experimental animals including monkeys, mice, cats, dogs, rats and goldfish.7 MPTP is a highly lipophilic molecule after systemic administration it crosses the blood-brain-barrier immediately. Within the brain, MPTP is rapidly converted to the hydrophilic metabolite 1-methyl-4-phenylpyridinium ion (MPP+)8 and is responsible for the dopaminergic neuron loss. MPTP was used in the current study to mimic PD in mice which was then subjected to neuroprotective treatment. Piper longum L. (PI Piperaceae) is also called as long pepper. It is a slender aromatic climber, is a native of the Indo-Malayan region and grows wild in the tropical rain forests of India.9 The extract of the crude drug “Piperis Longi Fructus,” the fruits of Piper longum (PI), is frequently used in folk medicine to treat various disorders.10-13 Piperine was the first amide isolated from Piper species and was reported to display central nervous system depression, antipyretic, and antiinflammatory activity. In the present study the neuroprotective action of PI was investigated on MPTP mouse model.
Methods
Healthy male Swiss albino mice (8-14 weeks old) 25-30g of weight were used throughout the study. Animals were purchased from Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Madhavaram, Chennai, India and maintained under constant temperature with free access to food (pellet diet Gold Mohor rat feed, Hindustan Lever Ltd., Bangalore, India) and water. This study was conducted according to the Animal Ethics Committee Guidelines of our Institution.
Chemicals
MPTP-HCI was purchased from Sigma-Aldrich, USA. Plant drug used for the study. Piper longum (PI) was obtained from local authorized dealers. All the chemicals used for the study were of analytical grade.
Experimental protocol
The experimental mice were divided into 4 groups with 6 animals in each group; group I received 0.9% NaCI (control); group II received MPTP-HCI at the dosage of 20 mg/kg of two doses at 2 h interval and served as PD model; group III received MPTP along with 50 mg/kg body weight of Piper longum (treatment group); group IV received 50 mg/kg body weight of Piper longum (drug control group). Animals were sacrificed on the 7th day of experimental protocol.
Tissue Collection
At the end of the experimental period, the rats were sacrificed by cervical decapitation. The brain tissue was excised immediately, rinsed in ice-cold saline, dried, weighed and homogenized in 0.1 M Tris-HCI buffer of pH 7.4 using a Teflon homogenizer. The tissue homogenate was then centrifuged in a refrigerated centrifuge at 1500 x g to remove the debris and the supernatant was analysed for various biochemical parameters. The tissue homogenate was stored at -20°C until further use.
Histopathological analysis
Mid portion of brain specimens obtained from all groups of animals were fixed in 10% formalin. The tissue sections were embedded in paraffin wax and sectioned at 5-6 μm thickness and sections were stained with haematoxylin-eosin for photo microscopic observations of the brain histopathological architecture.
Biochemical analysis
The protein content was determined by the method of Lowry et al.14 Superoxide dismutase was assayed following the method of Misra and Fridovich.15 Catalase was estimated as per the method of Takahara et al. 16 The level of lipid peroxides were estimated in mid brain by the method of Ohkawa et al.17 Reduced glutathione was studied by the method of Staal et al.18 Total ATPase activity was determined spectra metrically quantifying inorganic phosphorus produced by ATP hydrolysis. Inorganic phosphorus (Pi) was determined by a colorimetric assay of Taussky and Shorr;19 the activity of Mg2+ ATPase was determined by the method of White and Ralston.20 Ca2+ ATPase was determined by the method of Hejerten and Pan.21
Statistics
All the grouped data were evaluated with SPSS/10 software. Hypothesis testing methods included one way analysis of variance followed by Least Significant Difference test. P values of less than 0.05 were considered to indicate statistical significance. All these results were expressed as mean ±SE for six animals in each group.
Results
Fig. 1 illustrates the histopathological changes in control and experimental mice. Degenerative changes could be seen in MPTP mice, which included cellular inflammation, vascular degeneration and cytoplasmic vacuolation which can be altered on PI treatment.
Increased reactive oxygen species, formation and alteration of endogenous antioxidant enzyme activities were reported in the brains of MPTP-treated mice.22 Like these reports, the present study also showed a similar change of activities of antioxidant enzymes in the brain of mice treated with MPTP along with elevated LPO.
MPTP induced neurodegeneration and piper longum
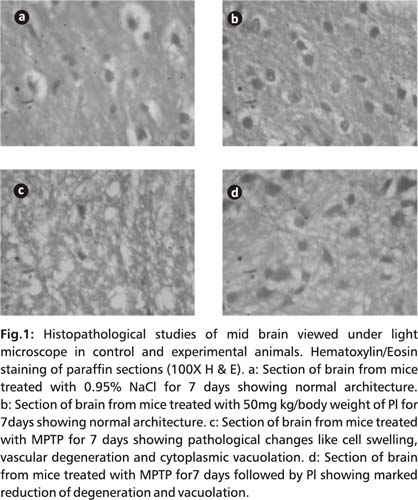
Table I shows antioxidant status; the activities of SOD, CAT and LPO in the midbrain were significantly elevated (p<0.05) and decreased GSH (p<0.05) in MPTP-treated animals (group II) relative to the controls. PI administration of MPTP-treated mice (group III) tends to bring the SOD and CAT close to normal values. No significant changes (p<0.05) were found in mice treated with PI alone (group IV).
Table 1: Oxidative stress levels in the brain of control and
experimental mouse.
| Groups | SOD Units/min/mg protein |
Catalase μMoles of H2O2 consumed/ min/mg protein |
LPO nmol of MDA released/ mg protein |
GSH g/mg protein |
| Group I | 1.55±0.31 | 0.98±0.10 | 23.31±0.54 | 2.54±0.27 |
| Group II | 3.41±0.45a | 2.70±0.35a | 64.96±0.81a | 1.28±0.18a |
| Group III | 1.57+0.18b | 1.05±0.34b | 24.04±0.75b | 2.49±0.36b |
| Group IV | 1.45+0.26ns | 1.20±0.32ns | 27.07±0.55ns | 3.41±0.57ns |
Statistical significance was represented as p<0.05. aGroup I were compared with Group I, & bGroup II were compared with Group III, ns-non significant. [Values are expressed as the mean ±SE]. Swiss albino male mice were treated with saline (0.95%) served as control. MPTP (20 mg/kg body weight 2 dose for 2 hr intervals) and Piper longum 50 mg/kg body weight. 6 animals treated in each group.
Table 2 shows activities of ATPases of brain tissue from experimental animals. The levels of these enzymes were reduced (p<0.05) in MPTP mice group when compared with control group. MPTP induced elevation of SOD, CAT were reduced almost to that of normal mice, while drug control group also showed similar values as that of control.
Table 2: Changes in the level of ATPase in the brain of control
and experimental mouse.
| Groups | Mg++ATPase | Ca++ATPase | Total ATPase |
| Group I | 4.86±1.72 | 2.26±1.35 | 6.41±1.67 |
| Group II | 1.63±1.13a | 1.72±1.36a | 3.25±2.26a |
| Group III | 4.67±1.94b | 2.42±1.76b | 5.68±2.29b |
| Group IV | 4.90±2.14ns | 2.50+1.66ns | 6.23±2.03ns |
Statistical significance was represented as p<0.05. Group I were compared with Group II & Group II were compared with Group III, ns-non significant. [Values are expressed as the mean ±SD].
Note : Swiss albino male mice treated with saline (0.95%) served as control. MPTP (20 mg/kg body weight 2 dose at 2 hr intervals) and Piper longum 50 mg/kg body weight. 6 animals treated in each group.
Discussion
Oxidative stress changes in the brain of Parkinson’s disease patients23, 24 and MPTP-mouse model and other animals are the importance of oxidative damage and the involvement of free radicals in the pathogenesis of this movement disorder.25-27
Inhibition of mitochondrial respiration in vivo and the generation of -OH dueto MPTP metabolites in rodents have been showed in the literature.28,29
There have been several reports during the past decade describing increased lipid peroxidation,30 a decrease in GSH content31, and changes in antioxidant enzyme activity32,33 in the brains of both parkinsonian patients and MPTP monkey models34-36 emphasising the importance of oxidative damage and the involvement of free radicals in the pathogenesis of this movement disorder.
The present study also supports these observations and provide a strong evidence for the involvement of -OH in the neurodegeneration caused by MPTP. A significant increase in the level of LPO was observed in the MPTP treated groups in mid brain when compared to control group. Lipid peroxidation induced by free radicals is believed to be one of the major causes of cell membrane damage leading to lysis of cell.37
SOD protects tissues by catalyzing the removal of superoxide radical that damage the membrane and its function. Catalase is responsible for the detoxification of significant amount of H202.38 MPTP mice showed increased activities of SOD, CAT in group II due to from the generation of OH to eliminate H202 formed in the brain39 in addition to the depletion of GSH.
The major observation of this study is the recovery of free radical induced damage in mid brain cells by PI. A significantly decreased activity of ATPase seen in MPTP mice caused due to the inhibition of complex I in the mitochondrial electron transport chain (induced by 1-methyl-4-phenylpyridinium (MPP+), results in impaired ATP production, loss of mitochondrial membrane potential, and formation of reactive oxygen species.40 The repeated administration of PI was found to reverse the influence of MPTP on SOD, CAT, GSH, LPO and ATPase activities.
The treatment of PD represents a significant challenge. In the later stages of PD, there is an urgent need for novel anti-PD treatment, without inducing severe drug-related involuntary movements. Finally, the challenge of developing an effective neuroprotective therapy for PD remains an exciting, if not elusive goal.
Thus, the treatment with PI holds promise to rectify the deleterious changes induced by MPTP. A detailed investigation about the exact role of PI against neurodegeneration needs further investigation.
Acknowledgements
We thank University Grants Commission for supporting the research fellowship, for the financial assistance in the form of Junior Research Fellowship.
Competing interests : None, Source of Funding - UGC
Received Date : 2 November 2009; Revised Date : 30 November 2009
Accepted Date : 15 December 2009
References
1.Isaacs T. The need for innovation in parkinson’s disease. Annals of Neurosciences.2009;16(4):146-147.
2.Langston JW, Ballard P, Tetrud JW et al. Chronic Parkinsonism in humans due to a product of meperidine. Analog synthesis 1983; 219: 979-980.
3.Vinish M and Milstein J. Non-motor aspects of Parkinson’s disease. Annals of Neurosciences 2009; 16(4): 176-179.
4.Agid Y Parkinson’s disease. Pathophysiology Lancet 1991; 337: 1321-1324.
5.Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated mouse model of Parkinsonism. J Neurosci Methods 2003; 123:189-200.
6.Oida Y, Kitaichi K, Nakayama H et al. Rifampicin attenuates the MPTP induced neurotoxicity in mouse brain. Brain Res 2006:1082:196-204.
7.Gerlach M, Riederer P, Przuntek H et al. MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur. J Pharmacol 1991;208:273-286.
8.Parmar VS, Jain SC, Bisht KS et al. Phytochemistry of the genus Piper. Phytochemistry 1997;46:597-673.
9.Singer TP, Ramsay RR. Mechanism of the neurotoxicity of MPTP An update. FEBS lett 1990;274:1 -8.
10.Singh MP, Agrawal V, Shukla R, et al. Probable toxin induced polyneuropathy. Case report of six individuals from a urban family of Lucknow. Annals of Neurosciences 2007;14(4):115-117.
11.Jung BS, Shin MK. Encyclopedia of Illustrated Korean Natural Drugs, Lim Sa, Seoul, 1989;818-819.
12.Lee EB, Shin KH, Woo W S. Pharmacological study on piperine. Arch Pharm Res 1984;7:127-132.
13.Shoba G, Joy D, Joseph T et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353-356.
14.Koul IB, Kapil A. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med 1993; 59:413-417.
15.Lowry OH, Rosenbrough NJ, Farr AL et al. Protein measurement with the Folin phenol reagent. J Bio Chem 1951; 193:265-276.
16.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Euro J Biochemistry 1974;47:469-474.
17.Sinha AK. Colorimetric assay of catalase. Anal Biochemistry 1972; 47:389-394.
18.Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochemistry 1979; 95:351-358.
19.Moron MS, Depierre JW, Mannervik B. Level of glutathione, glutathione reductase and glutathione- S-transferase activity in rat lung and liver. Biochem Biophys Acta 1979; 82:67-68.
20.Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. Journal of Biological Chemistry 1953; 202:675-685.
21.White MD, Rolston GB. A water soluble Mg2+ ATPase from erythrocyte membranes. Biochem Biophys Acta 1976; 436:567-576.
22.Vimal S, Sissodia SS, Meena P, et al. Antioxidant effects of asparagus racemosus wild and withania somnifera dunal in rat brain. Annals of Neurosciences 2005;12(4):67-70.
23.Amato RJ, Lipman ZP, Snyder S H. Selectivity of the parkinsonian neurotoxin MPTP: toxic metabolite MPP+ binds to neuromelanin. Science 1986; 231:987-989.
24.S. Patel, A. Sinha, D. Parmar, et al. An update on the role of environmental factors in parkinson’s disease. Annals of neurosciences 2005;12(4):79-86.
25.Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J Neurochem 1995; 64:919-924.
26.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Ann Rev Neurosci 1999; 22:123-144.
27.Kamal Saini, A Chaudhary and R K Sharma Biochemical estimation of antioxidant enzyme in cerebellum of ageing albino rats vis a vis effect of Celastrus paniculatus. Annals of Neurosciences 2009;16(3).
28.Obata, T, Chiueh CC. In vivo trapping of hydroxyl free radicals in the striatum utilizing intracranial microdialysis perfusion of salicylate: effects of MPTP, MPDP+ and MPP+. J Neural Transm 1992; 89:139-145.
29.Zang, LY, Misra HP. Generation of reactive oxygen species during the monoamine oxidase-catalyzed oxidation of the neurotoxicant 1 -methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. J Biol Chem 1993;268:16504-16512.
30.Dexter D, Carter C, Agid F et al. Lipid peroxidation as cause of nigral cell death in Parkinson’s disease. Lancet ii: 1986; 639-640.
31.Perry TL, Godin DV, Hansen S. Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 1982; 33:305-310.
32.Kish SJ, Morito C, Hornykiewicz O. Glutathione peroxidase activity in Parkinson’s disease brain. Neurosci Lett 1985; 58:343-346.
33.Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson’s disease. Arch Neurol 1975; 32:114-118.
34.Temlett JA, Landsberg P, Watt F et al. Increased iron in the substantia nigra compacta of the MPTP-lesioned hemiparkinsonian green monkey: evidence from proton microprobe elemental microanalysis. J Neurochem 1994; 62:134-146.
35.Mochizuki H, Imai H, Endo K et al. Iron accumulation in the substantia nigra of 1-methyl-4-phenyl-1,2,3.6-tetrahydropyridine (MPTP)induced hemiparkinsonian monkeys. Neurosci Lett 1994; 168:251-253.
36.Nakamura H, Kato S, Tanaka J. Mitochondria covered with a net of parallel and latticed filaments in nigral neurons of monkeys with experimental Parkinsonism. Acta Neuropathol 1989; 77:489-493.
37.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem 1992; 59:1609-1623.
38.Grankvist K, Marklund S, Sehlin J et al. Superoxide dismutase, catalase and scavengers of hydroxyl radicals protect against the toxic action of alloxan on pancreatic islets cells in vitro. J Biochem 1979; 182:17-25.
39.Chan P, DeLanney L E, Irwin I et al. Rapid ATP loss caused by 1 -methyl-4- phenyl-1,2,3,6-tetrahydropyridine in mouse brain. J Neurochem 1991;57:348-351.
40.Novelli A, Reilly J A, Lysko P G, et al. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced Brain. Res 1988; 451:205-212.
(c) Annals of Neurosciences.All Rights Reserved